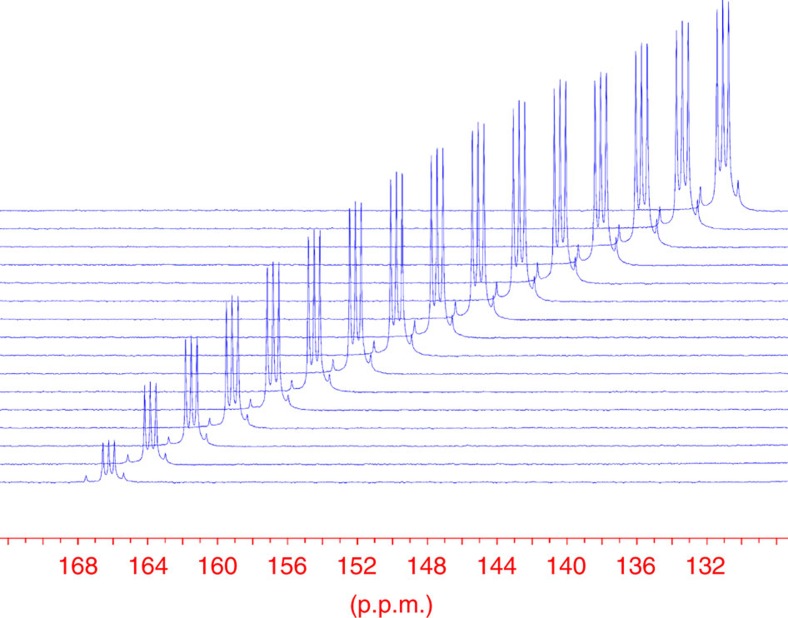
Figure 2. 13C NMR signals of DCOOD in the hydrogenation reactions of CO2 into formic acid in D2O.
[RuCl2(PTA)4] (2.76 mM) was dissolved in D2O (2 ml) under N2 atmosphere in a medium pressure sapphire NMR tube. This solution was pressurized at room temperature to 50 bar with CO2 and completed to 100 bar with H2. The system was heated to 60 °C and the reaction was followed by 13C NMR (100 MHz) spectroscopy. The figure shows the evolution of the 13C NMR signals of DCOOD at 166.3 p.p.m. (time difference between spectra Δt=189 min). The triplet signal of the formic acid is due to the exchange of the hydrogen to deuterium in HCOOH (as D2O was used as solvent). Reaction time: 148 h.