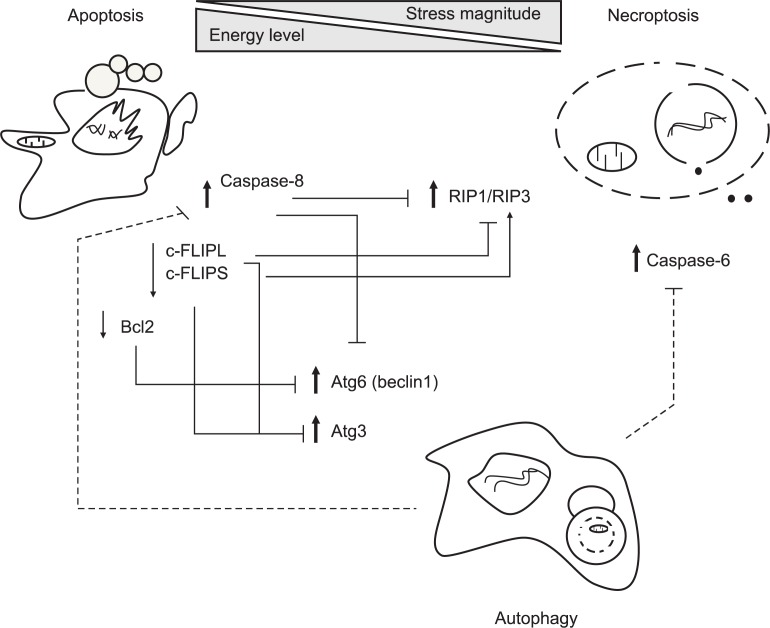
Fig. 2.
Crosstalk between apoptosis, necroptosis and autophagy. Cells determines actively cell death modes to external stresses through integrated decision of various factors including stress amplitude, cellular energy levels and induction of survival signals. Generally, controlled stimuli trigger default cell death apoptosis in a well-organized way while a specialized cell death necroptosis is activated under the condition when some death signals can induce apoptosis but a part of apoptotic machinery is defective. In aspects of energy crisis, apoptotic cell death occurs in the energy state sufficient to fuel caspase activity while necroptotic cell death prevails in the energy-deficient conditions, and autophagic suppression facilitates necroptosis. Occasionally, autophagy can be induced when default apoptosis is suppressed, but consequent effects depends on death contexts, leading to counteracting or promoting necroptosis. As for molecular regulation, apoptosis-associated proteins caspase-8 cleaves both RIP1 and RIP3 to abolish necroptotic downstream signals. Another antiapoptotic regulator c-FLIPL prevents formation of ripoptosome complex consisting of RIP, FADD and caspase-8/10 whereas c-FLIPS facilitates its assembly. Meanwhile, autophagy can also degrade caspase-8 to impede apoptosis although apoptosis reciprocally inhibits autophagy through Bcl-2 mediated sequestration and caspase-dependent degradation of Beclin-1. Autophagy can suppress necroptotic cell death by downregulation of C-6. Therefore, interplay between cell death modalities is so complicated that death fate will be determined depending on the different circumstances. Solid and dotted lines represent if associated proteins are identified or not. Also, bold and plain arrows indicate that specific proteins function positively and negatively on associated cell death, respectively.