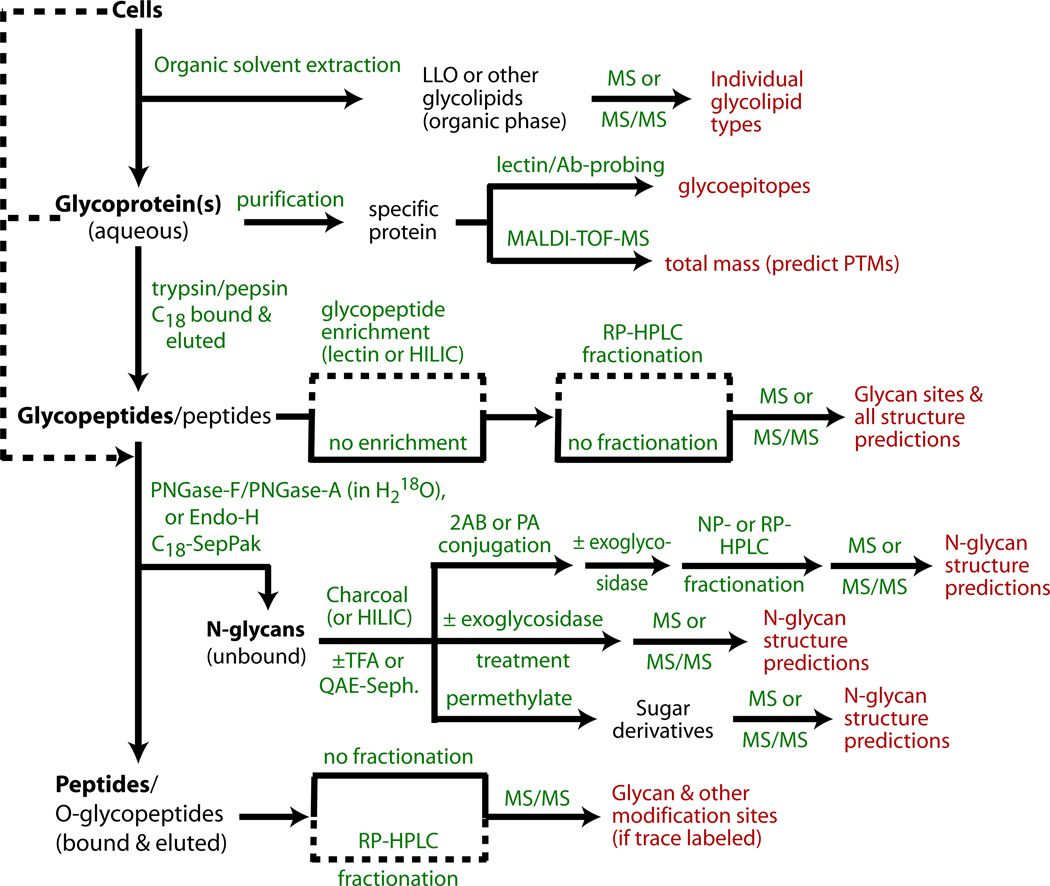
Figure 1. Flow chart for N-glycomic and N-glycoproteomic studies of cells and glycoproteins.
Cells are optionally initially extracted by organic solvent combinations to remove lipids and other small molecules (Subheading 3.1), and LLOs can be recovered. The remaining material can then be degraded by proteolysis to generate glycopeptides that can be analyzed for site-specific N-glycosylation (Subheading 3.13). Alternatively, purified glycoproteins can be analyzed for total mass and probed for lectin/Ab binding to query for discrete glycan structures (Subheading 3.16), and then introduced into the processing pipeline. Glycopeptides, which are more susceptible than intact glycoproteins to enzymatic de-N-glycosylation, are treated with PNGase F, PNGase A, or Endo-H to release N-glycans which are separated from peptides on a C18-SepPak (Subheading 3.3). The unbound glycans can be analyzed by MS in their native forms, before or after treatment with α-mannosidase or other exoglycosidases to probe the structure or non-reducing termini (Subheading 3.8). Alternatively, N-glycans are derivatized at their reducing termini with PA or 2AB (Subheading 3.6.1), and characterized chromatographically (Subheading 3.7), and/or analyzed by MS and MS/MS (Subheading 3.10) before or after permethylation (Subheading 3.6.2). If enzymatic de-N-glycosylation is performed in the presence of H218O, the peptides can be analyzed by MS for characteristic mass changes that allow confirmation of N-glycosylation (Subheading 3.15). Dashed lines indicate alternative paths or potential bypasses.