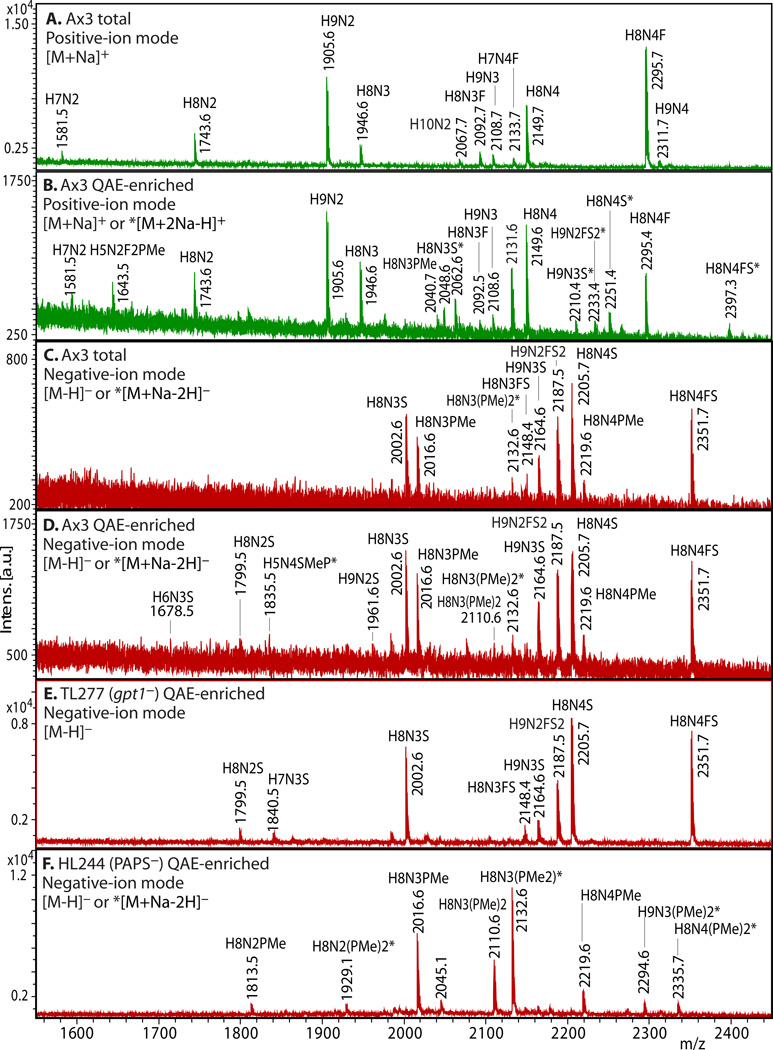
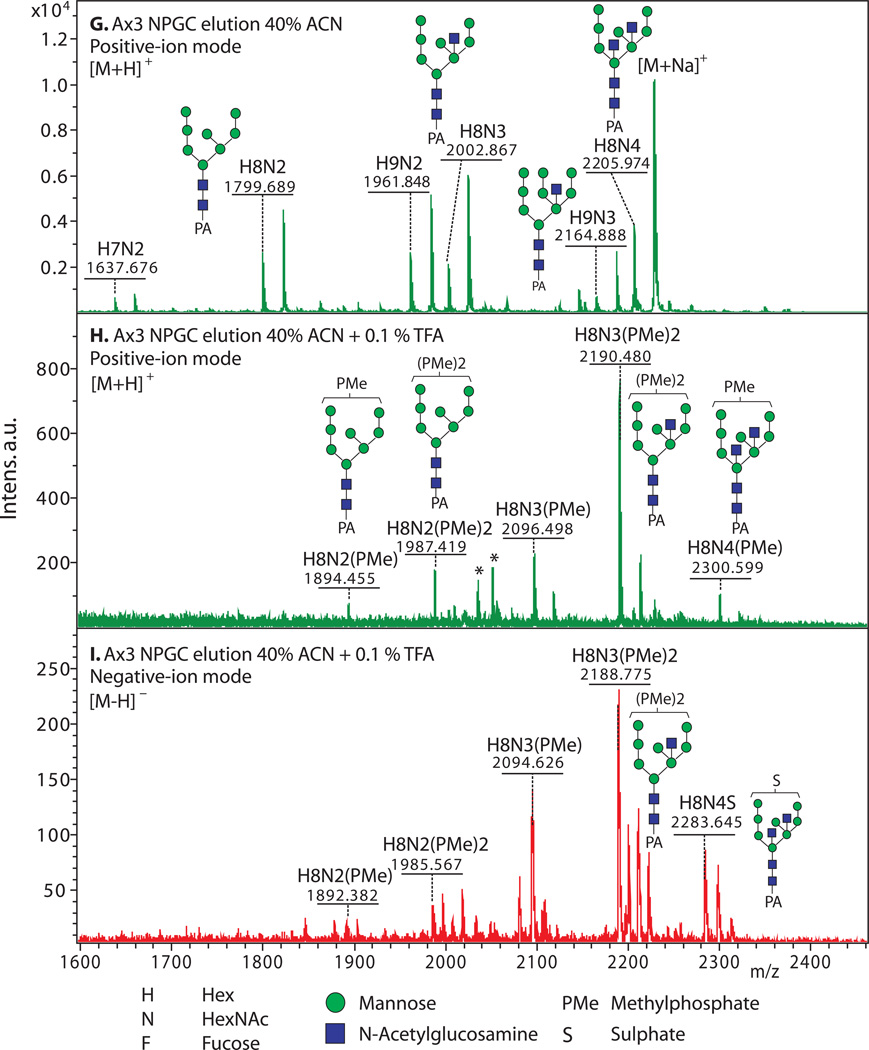
Figure 2. Enrichment of anionic N-glycans and analysis by MALDI-TOF-MS.
Enrichment based on anionic exchange (A–F) and porous graphitized carbon (G–I) are compared. (A–F) Total N-glycans were released from cells growing logarithmically in HL-5 axenic medium using PNGase A according to Subheading 3.3.2, and recovered according to Subheading 3.4. Anionic glycans were enriched using 70 mM NaCl according to Subheading 3.5.1, and MS-analyzed according to Subheading 3.10 using DHB. The underivatized native glycans were analyzed in standard positive-ion mode (green traces in panels A, B), or negative-ion mode (red traces in panels C-F) to enhance detection of anionic glycans. Glycan compositions (H=Hexose (Man); N=HexNAc or N-acetylhexosamine (GlcNAc); F=fucose(deoxyhexose); S=sulfate; PMe=methylphosphate) are assigned based on mass matching to glycan models (Subheading 3.11), with accuracy of 50 ppm, and confirmed (not shown) by MS/MS, α-mannosidase digestion (Subheading 3.8), and/or detection as their permethylated derivatives (Subheading 3.6.2.2). Structural models are illustrated in G–I. A) A mixture of high-mannose, β-GlcNAc-bisected and/or -intersected, ± core α3-fucosylated neutral species are revealed in the positive ion mode. Glycans ionize in their [M+Na]+ form. B) After anion exchange enrichment, mono-sulfated and mono-methylphosphorylated species are observed in the positive ion mode, in addition to carryover neutral species seen in panel A. Sulfated species appear in their [M+2Na-H]+ form under these conditions. C) Anionic species are selectively detected in negative ion mode analysis, as illustrated in this analysis of total glycans. Most ions occur in their [M–H]− forms, but di-methylphosphorylated glycans appear as their [M+Na–2H]− forms. D) Anion exchange enrichment typically enhances detection of anionic glycans in the negative ion mode, as suggested by evidence for additional low abundance species in this example. E) Similar analysis of strain TL277 (10), which is unable to form the methyl-phosphate substituent owing to the absence of gpt1, confirms assignments of the sulfated species in panel D. F) Similar analysis of strain HL244 (11), unable to form the sulfate substituent owing to a deficiency in PAPS (12), confirms assignments of the mono- and di-methylphosphorylated species. It is not known whether enhanced detection of the methylphosphorylated species is due to increased production in the absence of sulfation, or enhanced sensitivity in the absence of suppression by the sulfated species. (G–I) N-glycans were released using PNGase F from pepsin-glycopeptides from strain Ax3 (unicellular stage) (Subheading 3.3.3, steps 1–5). After adsorption to ENVI™ Carb NPGC material (Subheading 3.5.2), the neutral N-glycans (G) were eluted using ACN, and the acidic N-glycans with TFA/ACN (H, I). After separation, N-glycans were labeled with 2-aminopyridine (PA) (Subheading 3.6.1.2), and the glycans analyzed using MALDI-TOF-MS in positive- (G, H; green) or negative- (I; red) reflectron ion mode. The [M+H]+ ions are annotated with putative compositions and structures based on biosynthetic rules and MS/MS studies (not shown); sodium adducts [M+Na]+ are also present in the spectra. Peaks annotated with (*) are putatively not N-glycans. Further studies are needed to understand the reason for preferential detection of sulfated or methylphosphorylated N-glycans using the two methods, which were conducted on separate samples in different laboratories.