Abstract
Siberian hamsters (Phodopus sungorus) adapt to seasonal environmental conditions with marked changes in body mass, primarily in the form of adiposity. Winter-like conditions (e.g. short days) are sufficient to decrease body mass by approximately 30% in part via reductions in food intake. The neuroendocrine mechanisms responsible for these changes are not well understood, and homeostatic orexigenic/anorexigenic systems of the hypothalamus provide little explanation. We investigated the potential role of endocannabinoids, which are known modulators of appetite and metabolism, in mediating seasonal changes in energy balance. Specifically, we housed hamsters in long or short days for 0, 3, or 9 weeks and measured endocannabinoid levels in the hypothalamus, brainstem, liver and retroperitoneal white adipose tissue (RWAT). An additional group of males housed in short days for 25 weeks were also compared with long-day controls. Following 9 weeks in short days, levels of the endocannabinoid 2-arachidonoylglycerol (2-AG) were significantly elevated in RWAT and reduced in brainstem, although they returned to long-day levels by week 25 in short-day males that had cycled back to summer-like energy balance. Endocannabinoid levels in these tissues correlated significantly with adiposity and change in body mass. No photoperiodic changes were observed in the hypothalamus or liver; however, sex differences in 2-AG levels were found in the liver (males > females). We further tested the effects of CB1 receptor signalling on ingestive behaviour. Five daily injections of CB1 antagonist SR141716 significantly reduced food intake and body mass but not food hoarding. Although the CB1 agonist arachidonyl-2-chloroethylamide did not appreciably affect either ingestive behaviour, body mass was significantly elevated following 2 days of injections. Taken altogether, these findings demonstrate that endocannabinoid levels vary with sex and photoperiod in a site-specific manner, and that altered signalling at CB1 receptors affects energy balance in Siberian hamsters.
Keywords: energy balance, adiposity, seasonality, sex differences, 2-AG
Seasonally breeding animals respond to variable, yet predictable, environmental conditions with a wide range of physiological, morphological and behavioural adaptations that increase their chance of survival (1). One such species is the Siberian hamster (Phodopus sungorus), which must cope with relatively harsh winter conditions such as extreme temperatures and low food availability. Among several seasonally variable environmental factors, photoperiod (i.e. day length) is used as a noise-free cue of time of year and can be manipulated in laboratory settings to induce a suite of seasonal adaptations. For example, shortening photoperiods cue the onset of winter, and Siberian hamsters respond accordingly with gonadal regression, a thickened coat and substantial decreases in body mass. Reductions in body mass are achieved primarily through the depletion of fat stores and gradually reach 60–70% of summertime mass (2). Interestingly, prolonged exposure (approximately 25 weeks) to short photoperiods triggers a reversion to the summer phenotype with increased food intake and body mass (3).
Changes in body mass are driven in part by changes in food intake; however, the underlying neuroendocrine mechanisms for this are poorly understood. Orexigenic and anorexigenic genes in the hypothalamus are not likely responsible for seasonal changes in energy balance because either no changes are seen in their expression profiles across photoperiods or the direction of change is inconsistent with the photoperiodic phenotype. For example, hamsters acclimated to short photoperiods demonstrate decreased mRNA expression for anorexigenic pro-opiomelanocortin (4), corticotrophin-releasing hormone (5) and leptin receptor (5), whereas no changes are seen in mRNA for neuropeptide Y (4,5), orexin (5), melanin-concentrating hormone (5) or ghrelin (6). Moreover, up-regulation of orexigenic Agouti-related peptide by means of gene transfection does not prevent short-day decreases in body mass despite a 20% gain observed in long-day hamsters (7). The number of anorexigenic cocaine-amphetamine-regulated transcript-immunoreactive cells within the arcuate nucleus of the hypothalamus has been shown to increase in short photoperiods (8), consistent with the short-day phenotype, although whether this increase leads to functional catabolism remains to be investigated. The general lack of evidence implicating homeostatic peptides in the seasonal regulation of energy balance necessitates an investigation of alternative mechanisms.
Endogenous cannabinoids (i.e. endocannabinoids) are potential candidates because they modulate a variety of physiological functions, including appetite and energy balance. Central or peripheral administration of endocannabinoids (or their synthetic or plant-derived equivalents) results in hyperphagia, lipogenesis and an overall anabolic drive (9). Their orexigenic effects are mediated primarily through cognate CB1 receptors, as stimulated feeding is blocked by the selective CB1 antagonist rimonabant but not the CB2-selective antagonist SR144258 in rodent models (10). Endocannabinoid regulation of energy balance is further evidenced by studies in CB1 receptor knockout mice (CB1−/−). CB1−/−) mice demonstrate decreased adiposity and appetite compared with wild-type counterparts, and are resistant to diet-induced obesity, as well as diet-induced increases in hepatic lipogenesis and steatosis (11,12). Mice with selective deletion of CB1 from hepatocytes are also protected from diet-induced insulin- and leptin resistance, as well as from dyslipidaemia (13).
Together, these findings support endocannabinoid regulation of appetite and metabolism, but a role for endocannabinoids in seasonal regulation of energy balance is unknown. To address this, the present study tested the hypothesis that endocannabinoids respond to photoperiodic cues in Siberian hamsters with the potential to mediate seasonal variation in energy balance. In Study 1, we examined levels of the key, biologically-active endocannabinoids, anandamide and 2-arachidonoylglycerol (2-AG), in hamsters acclimated to long or short photoperiods in four metabolically-relevant tissues: the hypothalamus, brainstem, liver and retroperitoneal white adipose tissue (RWAT). The hypothalamus and brainstem are target sites for numerous metabolic peptides, whereas the liver is a primary source of fatty acid synthesis (14). In Siberian hamsters, RWAT is preferentially depleted in short days (15) and thus is particularly relevant to seasonal changes in energy balance. We further predicted that any photoperiod-driven divergence in endocannabinoid signalling would diminish as hamsters return to summertime energy balance because candidate mechanisms mediating seasonal energy balance should preferentially track energy status rather than photoperiod. Thus, we also examined males undergoing gonadal recrudescence following prolonged exposure to short photoperiods.
In Study 2, we determined the functional effects of CB1, the primary cannabinoid receptor through which changes in energy balance are achieved (9). Specifically, we tested the effects of CB1 stimulation or blockade on two important ingestive behaviours: food intake and food hoarding. Food intake directly affects energy balance, and Siberian hamsters often respond to metabolic challenges with changes in food hoarding but not food intake (e.g. following prolonged periods of food deprivation) (16). Thus, both are key components of the energetic budget in Siberian hamsters. We predicted that stimulation of CB1 receptors with a CB1-selective agonist would increase both food intake and food hoarding, whereas blockade of endogenous activity with a CB1-selective antagonist would result in a decrease in both behaviours.
As a final goal, we tested whether the endocannabinoid response to photoperiod and behavioural response to CB1 stimulation or blockade differ between sexes. Few studies have simulataneously examined both sexes despite the fact that food intake is a sexually dimorphic trait in rodents (17). Moreover, each sex is faced with particular energetic demands (e.g. pregnancy, ornamentation) that may well have shaped differences in their respective metabolic physiologies. Indeed, sex differences in endocannabinoid influence have been identified in other species. For example, in humans, males report higher subjective ratings of the effects of marijuana (18), and male rats are more sensitive to orexigenic effects of the CB1 receptor agonist CP 55 940 on the consumption of a palatable food (19). Sex steroids such as oestrogens and progesterone have been shown to regulate endocannabinoids (20,21) and may contribute to differential endocannabinoid signalling between sexes. To determine whether Siberian hamsters demonstrate sexual dimorphism in endocannabinoid signalling and in their response to altered activity at CB1 receptors, the present study was conducted in both sexes.
Materials and methods
Animals and housing
Adult Siberian hamsters (> 90 days old) were obtained from our laboratory breeding colony at Indiana University. Hamsters were bred and maintained under long days (LD; 16 : 8 h light/dark cycle; lights on 04.00 EST) with ambient temperatures of 20 ± 2 °C and relative humidity at 50 ± 5%. Hamsters were given ad lib. access to standard laboratory rodent chow and tap water throughout the experiment except where noted in the Experimental design. All procedures were approved by the Bloomington Institutional Animal Care and Use Committee (BIACUC).
Study 1: Endocannabinoid signalling across photoperiod and sex
Experimental design
Hamsters were individually housed in polypropylene cages (27.8 × 17.5 × 13.0 cm) 1 week before the start of experiments. Animals were randomly assigned to experimental groups (n = 6 per group) that were subsequently equalised by body mass within each sex. Hamsters were then transferred to their respective experimental housing rooms and maintained in long or short days (SD; 8 : 16 h light/dark cycle; lights on 08.00 h) with ambient temperature and relative humidity kept the same as with colony conditions. Body mass and food intake were measured weekly between 08.00 h and 10.00 h until euthanasia at 0, 3, or 9 weeks. These time points were chosen to capture baseline levels and points of initial and final adaptation to short photoperiods, respectively. Additional males were housed in long or short photoperiods for 25 weeks to determine whether any seasonal changes in endocannabinoid levels are reversed in animals that have cycled back to summertime energy balance (e.g. increased body mass, increased food intake) and were used in this study to separate changes in energy balance from photoperiod. Gonadal recrudescence was used as an additional biological marker of the summer phenotype and was defined in the present study as paired testis mass weighing > 0.2 g.
Collection of tissue samples
Necropsies were performed beginning at 08.00 h for long-day animals and 12.00 h for short-day animals (i.e. 4 h after lights-on in each photoperiod). Animals were overdosed with i.p. injections of a ketamine cocktail comprised of ketamine (100 mg/ml; Henry Schein, Melville, NY, USA), xylazine (100 mg/ml; Henry Schein), and 0.9% saline and were deeply anaesthetised within 2 min. Brain and peripheral tissues were collected, weighed and flash frozen in liquid nitrogen before being stored at −80 °C. The hypothalamus was isolated by exposing the ventral side of the brain and dissecting caudal to the optic chiasm and rostral to the midbrain. Brainstem samples were isolated via a coronal cut just rostral and caudal to the cerebellum and extracting the brainstem segment beneath the cerebellum.
Compound extraction from tissue samples
Tissues were processed based as described previously (22). Briefly, tissues samples (approximately 0.1 g for liver and RWAT; the entire sample for hypothalamus and brainstem) were incubated on ice with 50 volumes of 100% high-performance liquid chromatography (HPLC)-grade methanol for 1 h. One hundred picomol [2H8]-anandamide (Cayman Chemical, Ann Arbor, MI, USA) was added to the methanol/tissue sample and used as an internal standard to track the recovery of the test compounds. Following incubation, samples were maintained on ice and homogenised via a polytron for 1–2 min. Samples were centrifuged at 42 858 g and 24 °C for 20 min. Supernatants were collected and diluted to a 25% organic solution with HPLC-grade water. Compounds were partially purified from the supernatant using 500 mg C18 solid phase extraction columns (Varian, Harbor City, CA, USA), conditioned with 5 ml pf 100% HPLC-grade methanol and 2.5 ml pf HPLC water before the addition of the water/supernatant solution. Columns were washed with 2.5 ml of HPLC-grade water and three elutions were collected (1.5 ml each of 65%, 80% and 100% HPLC-grade methanol). Elutions were stored at −20 °C until mass spectrometric analysis. Each tissue type was processed separately with all experimental groups analysed within each batch.
HPLC/MS/MS analysis and quantification
Each elution was warmed to room temperature and vortexed before mass spectrometric analysis. Rapid separation was obtained with 10–20 µl injections of analyte (CBM-20A prominence controller and SIL-20AC prominence autosampler; Shimadzu, Columbia, MD, USA; maintained at 24 °C) onto a Zorbax eclipse XDB 2.1 × 50 mm reversed phase column (Agilent Technologies Inc., Santa Clara, CA, USA). Gradient elution (200 µl/min) was formed under pressure on a pair of 10AdVP pumps (Shimadzu). Mass spectrometric analysis was performed using electrospray ionisation with a triple quadropole mass spectrometer (API3000; Applied Biosystems/MDS Sciex, Foster City, CA, USA). Levels of each compound were analysed by multiple reactions monitoring on the HPLC/MS/MS system. In this mode, the detection of each compound is based on fragmentation of a precursor ion [M+H]+ or [M−H]+ to yield a prominent product ion. Mass spectrometric conditions were optimised for each compound using direct flow injection of synthetic standards of each compound. Test compounds of interest were the endocannabinoids 2-AG and anandamide. Structurally-related N-acyl ethanolamides palmitoylethanolamide (PEA), oleoylethanolamide (OEA), linoleoylethanolamide (LEA) and stearoylethanolamide (SEA) were also examined to assess whether differences in endocannabinoid signalling are ligand-specific or part of a global change in lipid expression.
Study 2: Effects of CB1 stimulation or blockade on ingestive behaviours
Experimental design
As with Study 1, hamsters were acclimated to individual housing for 1 week under similar conditions as described above, then weighed and assigned to experimental groups equalised by body mass within each sex. Hamsters were then maintained in LD or SD for 8 (males) or 9 (females) weeks, followed by another week-long acclimation period in the hoarding apparatus (see Hoarding apparatus below).
Baseline measures of food intake and food hoarding were taken daily for 1 week following the acclimation period to the hoarding apparatus. During this time, body mass was measured every 3 days. Standard laboratory chow was available in cage top hoppers on both levels of the apparatus, as well as in pelleted form in the upper cage, during the acclimation period. Within each sex and photoperiod, hamsters were assigned to one of four drug treatment groups equalised by post-acclimation body mass and food intake.
Hamsters received 0.3-ml i.p. injections of each drug or its vehicle once daily for 5 days, allowed a 5-day washout period, followed by an additional 5 days of daily injections. Drug treatment was counterbalanced such that each individual served as its own control: half the animals received the agonist or antagonist first, whereas the other half started with its corresponding vehicle. After the washout period, treatment was reversed; hamsters that had received drug first were now given its corresponding vehicle, and vice versa. Throughout this period, food intake and food hoarding were measured daily 1 h after lights-on in each photoperiod. Body mass was measured every 3 days at the same time. The amount of food hoarded was determined by taking the summed weight of any food found in the lower cage and that found in the hamster’s cheek pouches. Food intake was calculated by subtracting the sum of food hoarded and the amount remaining in the upper cage from the total weight of food provided the previous day. Hoards were discarded after they were found and a new allotment of food was weighed and replenished.
Hoarding apparatus
Owing to the predictability and proximity of food in cage top hoppers, Siberian hamsters typically do not hoard food in standard rodent cages, despite being reported to do so in their natural environment (23). Thus, a hoarding apparatus was specially constructed to facilitate this behaviour. The design of the hoarding apparatus was originally based on those described previously (24) and has been since adapted in our laboratory (25). The hoarding apparatus consisted of two polypropylene standard small rodent cages (29.2 × 19.1 × 12.7 cm), positioned one above the other on two levels of a cage rack. The cages were connected with 1.5-in polyvinylchloride tubing approximately 55 cm in length, with one end exiting the front of the upper cage and the other entering the front of the lower cage to form a C-shape structure. The tubing portion included corners and straight-ways for horizontal and vertical components, and were lined with aluminum mesh material to aid in vertical ascents. The bottom cage served to simulate the ‘home burrow’ and contained standard bedding material used in the colony (Sani-Chips; P. J. Murphy Forest Products, Montville, NJ, USA), cotton bedding material and water. Approximately 50 g of 97-mg food pellets (Grain-based rodent tablet; TestDiet, Richmond, IN, USA) was supplied daily in a shallow metal food dish placed in the upper cage to simulate the ‘foraging environment’. Although the pellet formula is identical to their standard block chow, its size allows hamsters to transport whole pellets in their cheek pouches to cache in their home burrows.
Drug preparation
The CB1-selective agonist arachidonyl-2-chloroethylamide (ACEA; Tocris Bioscience; Ellisville, MO, USA) arrived predissolved in anhydrous ethanol and was diluted with 0.9% saline to a concentration of 0.25 mg/kg. Its corresponding vehicle was a solution that consisted of 10% ethanol in 0.9% saline. The CB1-selective antagonist SR141716 (rimonabant) was provided by the National Institute on Drug Abuse (NIDA) program, dissolved in 1% dimethyl sulphoxide (DMSO; Tocris Bioscience), and diluted to 3 mg/kg in 0.9% saline. All solutions were prepared on the first day of injection and used throughout the 5-day treatment period, except for agonist solutions, which were prepared fresh daily.
Statistical analysis
Differences in group means were analysed in spss, version 17 (SPSS, Inc., Chicago, IL, USA) statistical software using three-way anova followed by post-hoc unpaired or one-sample t-tests where significance was achieved. Where noted, data that were not normally distributed were log10-transformed to reduce heterogeneity of variance and statistical analyses were conducted on transformed data. In Study 1, unpaired t-tests were used for measures of body mass, tissue mass and food intake. Pearson correlation coefficients were calculated between endocannabinoid levels and measures of energy balance. Data points were considered outliers and removed from statistical analysis if they fell beyond 2 standard deviation. Thus, data were omitted for 1 SD female for RWAT anandamide, 2 SD females for RWAT 2-AG and 1 SD male for brainstem 2-AG.
Because injections themselves affected our measures of interest and catabolic responses to short photoperiods were still underway at the time of testing, a composite score was created for each measure to take these factors into account. To determine the effects of drug treatment on ingestive behaviours, mean daily food intake or hoard following vehicle injections was subtracted from their mean daily food intake or hoard following drug injections. To determine the effects of drug treatment on body mass, a repeated-measures anova was used for statistical analysis. Change in body mass was calculated by subtracting body mass following days 2 and 5 of vehicle injections from body mass taken on corresponding days of drug injections. Because variances were unequal across samples, body mass data were log10-transformed following the addition of a constant (as a result of negative values), and outliers that fell beyond 2 standard deviation were removed. This resulted in the omission of data from two SD males following injection 2, and three LD males, one SD male and one LD female following injection 5. Two SD males lost an excessive amount of body mass as a result of a drop in food intake and died during the course of Study 2. This is unlikely due to drug effects as both animals died following the washout period during vehicle injections. Their data have also been excluded from analyses. Values are expressed as the mean ± SEM. P < 0.05 was considered statistically significant.
Results
Hamsters lose body mass and decrease food intake in short photoperiods
As expected, exposure to short photoperiods led to significant reductions in body mass and food intake. Body mass decreased steadily and significantly following 3 weeks of exposure to short photoperiods in males (P < 0.05 for all time points; Fig. 1a). Females demonstrated reductions in body mass around the same time as males; however, these were not statistically significant until week 6 (P < 0.05 for all time points; Fig. 1b). Food intake was similarly affected and coincided with changes in body mass. Although differences in food intake were significant at week 7 in males and week 4 in females (Fig. 1c,d), initial changes in food intake began around the same time as those observed for body mass.
Fig. 1.
Body mass (a, b) and weekly food intake (c, d) in response to long (open circles; 16 : 8 h LD) or short (closed circles; 8 : 16 h LD) photoperiods. Data are presented as the mean ± SEM. *P < 0.05, t-test (n = 6 per group).
Gonad and RWAT mass are reduced in short photoperiods
Reductions in body mass were in part due to decreased gonad and RWAT mass, which demonstrated a significant three-way interaction between photoperiod, sex and week (F1,50 = 7.89, P = 0.007; statistics conducted on log-transformed data) and a significant photoperiod by week interaction (F1,50 = 12.61, P = 0.001), respectively. In long photoperiods, male gonad mass remained relatively stable across time (week 0: 0.78 ± 0.07 g; week 9: 0.82 ± 0.05 g; week 25: 0.83 ± 0.07 g) but regressed significantly in short photoperiods (week 9: 0.07 ± 0.01 g). By week 25, short-day male gonads had returned to an intermediate mass of 0.49 ± 0.09 g. As with males, female gonad mass remained stable in long photoperiods (week 0: 0.13 ± 0.02 g; week 9: 0.16 ± 0.03 g) but decreased substantially in short photoperiods (week 9: 0.04 ± 0.002 g). Similarly, RWAT mass was significantly lower following 9 weeks in short days in both males (week 0: 0.21 ± 0.03 g; long day week 9: 0.25 ± 0.03 g versus short day week 9: 0.06 ± 0.01 g) and females (week 0: 0.24 ± 0.03 g; long day week 9: 0.25 ± 0.03 g versus short day week 9: 0.06 ± 0.02 g). Although RWAT mass was decreased to 0.14 ± 0.03 g by week 25 in long days, RWAT mass was comparable to long-day levels in short days (0.16 ± 0.05 g).
Endocannabinoid levels change in response to photoperiod
A main effect of photoperiod (F1,49 = 5.95, P = 0.018) was found in brainstem 2-AG levels, with a trend for an interaction between photoperiod and week (P = 0.063). Levels of 2-AG significantly decreased following 9 weeks in short days, although this effect was only observed in males (Fig. 2; see also Supporting information, Table S1). Importantly, brainstem 2-AG returned to levels comparable to those of long-day controls following 25 weeks. A positive correlation with change in body mass across the 9-week period (r = 0.413, P = 0.050; Fig. 3a) was also observed. A similar correlation was seen with RWAT mass (r = 0.331, P = 0.010; Fig. 3b), although no differences were found in levels of anandamide in the brainstem (P > 0.05; data not shown).
Fig. 2.
Brainstem levels of 2-arachidonoylglycerol (2-AG) in males (a) and females (b) in response to long (white bars; 16 : 8 h LD) or short (black bars; 8 : 16 h LD) photoperiods. Data are presented as the mean ± SEM. *P < 0.05, t-test (n = 5–6 per group).
Fig. 3.
Retroperitoneal white adipose tissue (RWAT) levels of 2-arachidonoylglycerol (2-AG) in males (a) and females (b) in response to long (white bars; 16 : 8 h LD) or short (black bars; 8 : 16 h LD) photoperiods. Data are presented as the mean ± SEM. *P < 0.05, t-test (n = 5–6 per group).
In RWAT, 2-AG was present at much lower concentrations than in brainstem. A significant interaction between photoperiod and time for RWAT 2-AG was observed (F1,50 = 43.17, P < 0.001; statistics conducted on log-transformed data), although in a manner opposite to that seen in the brainstem. In RWAT, 2-AG was significantly elevated following 9 weeks in short photoperiods in both males and females, and returned to similar levels as long-day controls by week 25 (Fig. 4; see also Supporting information, Table S2). These levels correlated negatively with change in body mass over the course of 9 weeks (r = −0.747, P < 0.001; statistics conducted on log-transformed data; Fig. 3c) and RWAT mass (r = −0.565, P < 0.001; statistics conducted on log-transformed data; Fig. 3d). By contrast, anandamide correlated positively with RWAT mass (r = 0.308, P = 0.018; data not shown) and change in body mass across 9 weeks (r = 0.547, P = 0.006; Fig. 3b). Thus, short-day animals demonstrating the greatest reductions in body mass and adiposity had higher levels of 2-AG and lower levels of anandamide in RWAT.
Fig. 4.
Relationships between 2-arachidonoylglycerol (2-AG) levels and change in body mass following the initial 9-week period in the brainstem (a) and retroperitoneal white adipose tissue (RWAT) (b) of male and female hamsters (n = 23). Relationships between brainstem (c) and RWAT (d) 2-AG levels and RWAT mass of male and female hamsters (n = 59 for brainstem, 58 for RWAT). P < 0.05, Pearson’s correlation coefficient.
No main effects of photoperiod were seen in the endocannabinoid content of the hypothalamus (see Supporting information, Table S3) or liver (see Supporting information, Table S4), nor in the related lipid signalling molecules LEA, OEA, PEA and SEA in any of the tissues examined (P > 0.05; see Supporting information, Tables S1–S4). However, the change in body mass correlated negatively with 2-AG in the liver (r = −0.463, P = 0.023; data not shown) and males displayed significantly higher levels of 2-AG in the liver than did females (6.05 ± 0.42 versus 4.26 ± 0.23 nmol/g; F1,50 = 13.67, P = 0.001).
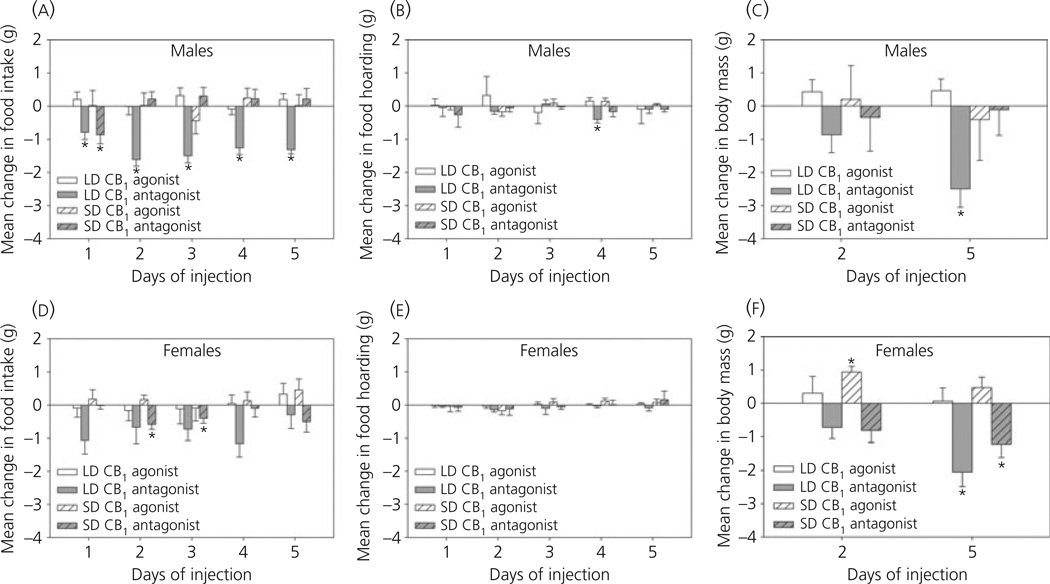
Blockade of CB1 receptors decreases food intake but not food hoarding
Significant main effects of photoperiod (F1,56 = 15.104; P < 0.001) and drug treatment (F1,56 = 32.854; P < 0.001) were observed in food intake following 5 days of drug treatment. Treatment with the CB1-selective agonist did not significantly affect food intake; however, the CB1-selective antagonist SR141716 significantly reduced food intake in males and females. A significant interaction between photoperiod, sex and drug treatment (F1,56 = 6.481; P = 0.014) revealed this effect was only significant in LD males (t7 = −11.378, P < 0.001; Fig. 5a), whereas the CB1 antagonist significantly decreased food intake in both LD (t7 = −4.761, P = 0.002) and SD females (t9 = −2.456, P = 0.036; Fig. 5d). By contrast, food hoarding was not appreciably affected by either drug (P > 0.05; Fig. 5b,e).
Fig. 5.
Changes in food intake (a, d), food hoarding (b, e), and body mass (c, f) following daily treatment with CB1-selective agonist arachidonyl-2-chloroethylamide (0.25 mg/kg; white bars) or CB1-selective antagonist SR141716 (3 mg/kg; grey bars). Changes were calculated by subtracting mean values following vehicle injections from mean values following drug treatment. Data are presented as the mean ± SEM. *P < 0.05, t-test (n = 7–10 per group).
Altered CB1 signalling affects body mass
A significant effect of drug treatment on body mass was observed (F1,50 = 10.802; P = 0.002) with similar responses across sex and photoperiod. Treatment with the CB1 agonist resulted in a significant gain in body mass after 2 days, although these differences were no longer significant following 5 days of treatment (Fig. 5c,f). By contrast, significant reductions in body mass were observed at both times of measurement following treatment with the antagonist (2 days, t32 = −2.584, P = 0.015; 5 days, t32 = −5.091, P < 0.001; Fig. 5c,f).
Discussion
The present study investigated whether endocannabinoid signalling varies seasonally and between sexes, with the broader aim of determining whether such changes contribute to seasonal energy balance in Siberian hamsters. We found that brainstem levels of the endocannabinoid 2-AG decreased following 9 weeks of exposure to short photoperiods, although only to a significant extent in males. However, these changes appeared to reflect the energetic status of the individual rather than photoperiod itself; differences in 2-AG levels were no longer present by week 25 when short-day males had regained appetite and body mass. Moreover, brainstem 2-AG levels correlated positively with gains in body mass and with RWAT mass in both males and females.
The caudal brainstem is a critical site for the autonomic control of appetite, and neurones in brainstem regions such as the dorsal vagal complex regulate intake, digestion and absorption of food (26). These neurones maintain the ability to respond to satiety signals and decrease food intake in the absence of connections to the forebrain (27). Given that endocannabinoids modulate neuronal signalling through retrograde inhibition of ongoing transmitter release (28), a decrease in brainstem 2-AG may result in a strengthened satiety signal. This would be consistent with the short-day decreases in appetite and body mass observed in Siberian hamsters, as with the finding that obese Zucker rats demonstrate elevated levels of CB1 mRNA in the dorsal vagal complex (29). However, the functional effects of brainstem 2-AG signalling and why female hamsters do not also demonstrate short-day decreases in brainstem 2-AG are currently unclear and require further testing.
By contrast, patterns of RWAT endocannabinoid response to photoperiod were opposite to those in the brainstem. 2-AG levels in this fat pad increased following 9 weeks in short photoperiods, returned to long-day levels by week 25 and correlated negatively with adiposity and change in body mass. Again, these findings demonstrate that 2-AG signalling mirrors the energetic phenotype rather than photoperiod per se. As before, the functional significance of these changes is currently unclear; however, activation of CB1 receptors in adipocytes results in anabolic processes such as increased lipoprotein lipase activity and glucose intake to promote lipogenesis (30). These findings sit in contrast to the low adiposity levels of short-day hamsters but, as with other peptide systems studied to date, the short-day increases in RWAT 2-AG may reflect a response to ongoing catabolism. Moreover, our findings in RWAT are similar to other findings demonstrating decreased levels of 2-AG in subcutaneous adipose tissue of obese patients with type II diabetes relative to normal weight controls (31). In this and the present study, levels of 2-AG in adipose tissue inversely relate to the energy status of the individual.
Because changes in food intake and body mass preceded those observed for endocannabinoid signalling, our results suggest that differences in 2-AG are likely a consequence (and not a cause) of seasonal changes in energy balance. However, an elicited response in endocannabinoid signalling is still a change in physiology that may affect downstream mechanisms relevant to the long-term maintenance of energy balance. Importantly, we found that blockade of endogenous signalling at CB1 receptors results in decreased food intake and body mass. In females, mean food intake was significantly reduced over the 5-day treatment period regardless of photoperiod. In males, this effect was only sustained in long days, albeit to a greater extent than in females.
Interestingly, this interaction between sex and photoperiod reflects that observed in brainstem 2-AG signalling and could explain why feeding responses to CB1 antagonism are differentially expressed between sexes. In females, endocannabinoid levels do not fluctuate considerably across photoperiods; thus, blockade of cognate receptors would affect food intake to the same degree. In males, however, endogenous ligand levels are already reduced in short days, thereby decreasing the possible magnitude of effect of a receptor antagonist. Although this hypothesis remains speculative and rests on the assumption that brainstem CB1 receptors are involved in mediating food intake responses, seasonal changes in 2-AG or even CB1 levels are a plausible mechanism for the observed responses and warrant further investigation.
In addition, body mass was transiently, yet significantly, elevated following 2 days of treatment with the CB1 agonist and significantly reduced during both sampling times with the CB1 antagonist. These responses did not differ significantly across sex or photoperiod. Given that food intake was not appreciably altered by the agonist, the observed increase in body mass is likely a result of changes in other factors such as energy expenditure or food assimilation. Indeed, chronic CB1 antagonism in rats increases temperature and uncoupling protein in brown adipose tissue, thereby implicating an effect of endocannabinoids on energy expenditure (32). Surprisingly, no differences were observed in hypothalamic endocannabinoid content in response to photoperiod or sex. However, the hypothalamus is comprised of a diverse group of nuclei that differentially affect energy balance. Any seasonal modulation that may take place at the level of the nucleus would not have been detected by measures used in the present study. Although CB1 receptor levels do not change seasonally in individual hypothalamic nuclei (J. M. Ho and G. E. Demas, unpublished data), this does not rule out the possibility for nucleus-specific changes in endocannabinoid levels. Specificity in modulation of endocannabinoid signalling may also come at the level of neuronal population type and should be addressed in future studies.
As with the hypothalamus, no photoperiodic differences were observed in the liver. However, males demonstrated significantly higher levels of 2-AG compared to females and levels of 2-AG correlated negatively with change in body mass. Although the scope of the present study does not address the functional implications of these findings, endocannabinoid signalling clearly varies in ligand-, sex- and site-specific ways. Different or even opposing patterns of anandamide and 2-AG have previously been reported across physiological conditions in humans and laboratory animals, suggesting that these endocannanbinoids serve separate functions (33). Moreover, the present findings suggest that brainstem, rather than hypothalamic, circuits, may be more relevant to seasonal changes in energy balance. Although numerous hypothalamic peptide systems do not fluctuate seasonally, photoperiodic changes in the satiety signals corticotrophin-releasing hormone and neurotensin have previously been reported in the brainstem of Siberian hamsters (34). In Siberian hamsters, seasonal changes in body mass and adiposity are supported by changes in energy intake and expenditure (35,36). Thyroid hormone signalling appears to gate seasonal reproduction and body mass in Siberian hamsters (37), and photoperiodic response of energy-relevant genes such as those for the histamine H3 receptor, vascular growth factor and retinoic acid signalling pathways has previously been reported (38–40). The present study aimed to assess whether endocannabinoids also demonstrate seasonal variation with energy balance. We found that central and peripheral endocannabinoids respond to sex and photoperiod and correlate with measures of energy balance. Moreover, we found that blockade of endogenous activity at CB1 receptors decreases body mass and food intake in male and female hamsters. Although food hoarding was not significantly affected by these manipulations, hoarding responses in Siberian hamsters are not typically photoperiod-dependent and are likely mediated by a separate set of physiological mechanisms (16).
Taken altogether, our findings provide the first demonstration of endocannabinoid seasonality in Siberian hamsters and corroborate the previously established complexity of endocannabinoid signalling and function. While several physiological systems have now been shown to be seasonally responsive, determining their relative contribution to functional metabolic change will greatly expand our understanding of seasonal energetics. Given the importance of such a function, it is likely that multiple regulatory pathways act in concert to orchestrate seasonal changes in energy balance. The present findings demonstrate that endocannabinoids vary with energetic status and carry the potential to affect food intake and body mass in Siberian hamsters. Despite a lack of support for their involvement in initiating seasonal changes in energy balance, our findings demonstrate that CB1 blockade differentially affects food intake across photoperiods and opens the possibility for a role maintaining long-term seasonal changes in energy balance.
Supplementary Material
Acknowledgements
We thank Emily Chester, Amy Sutton and Sucharat Tayarachakul for their technical assistance, as well as Jordyn Stuart for technical training. Funding was provided by NSF grant IOB-053798 to G.E.D., NIH grant DA018224 to H.B.B., and a fellowship from the Center for the Integrative Study of Animal Behavior to J.M.H.
Footnotes
Supporting information
The following supplementary material is available:
Table S1. Brainstem levels (nmol/g) of endocannabinoids and N-acyl ethanolamides.
Table S2. Retroperitoneal white adipose tissue levels (nmol/g) of endocannabinoids and N-acyl ethanolamides.
Table S3. Hypothalamic levels (nmol/g) of endocannabinoids and N-acyl ethanolamides.
Table S4. Liver levels (nmol/g) of endocannabinoids and N-acyl ethanolamides.
This supplementary material can be found in the online article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Nelson RJ, Badura LL, Goldman BD. Mechanisms of seasonal cycles of behavior. Annu Rev Psychol. 1990;41:81–108. doi: 10.1146/annurev.ps.41.020190.000501. [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Wade GN. Photoperiodic control of seasonal body-weight cycles in hamsters. Neurosci Biobehav Rev. 1985;9:599–612. doi: 10.1016/0149-7634(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 3.Masuda A, Oishi T. Effects of restricted feeding on the light-induced body-weight change and locomotor-activity in the djungarian hamster. Physiol Behav. 1995;58:153–159. doi: 10.1016/0031-9384(95)00035-h. [DOI] [PubMed] [Google Scholar]
- 4.Reddy AB, Cronin AS, Ford H, Ebling FJ. Seasonal regulation of food intake and body weight in the male Siberian hamster: studies of hypothalamic orexin (hypocretin), neuropeptide Y (NPY) and pro-opiomelanocortin (POMC) Eur J Neurosci. 1999;11:3255–3264. doi: 10.1046/j.1460-9568.1999.00746.x. [DOI] [PubMed] [Google Scholar]
- 5.Mercer JG, Moar KM, Ross AW, Hoggard N, Morgan PJ. Photoperiod regulates arcuate nucleus POMC, AGRP, and leptin receptor mRNA in Siberian hamster hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2000;278:R271–R281. doi: 10.1152/ajpregu.2000.278.1.R271. [DOI] [PubMed] [Google Scholar]
- 6.Tups A, Helwig M, Khorooshi RM, Archer ZA, Klingenspor M, Mercer JG. Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus) J Neuroendocrinol. 2004;16:922–928. doi: 10.1111/j.1365-2826.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 7.Jethwa PH, Warner A, Fowler MJ, Murphy M, de Backer MW, Adan RA, Barrett P, Brameld JM, Ebling FJ. Short-days induce weight loss in Siberian hamsters despite overexpression of the Agouti-related peptide (AgRP) gene. J Neuroendocrinol. 2010;22:564–575. doi: 10.1111/j.1365-2826.2010.02001.x. [DOI] [PubMed] [Google Scholar]
- 8.Khorooshi R, Helwig M, Werckenthin A, Steinberg N, Klingenspor M. Seasonal regulation of cocaine- and amphetamine-regulated transcript in the arcuate nucleus of Djungarian hamster (Phodopus sungorus) Gen Comp Endocrinol. 2008;157:142–147. doi: 10.1016/j.ygcen.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham TC. Endocannabinoids in the regulation of appetite and body weight. Behav Pharmacol. 2005;16:297–313. doi: 10.1097/00008877-200509000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Williams CM, Kirkham TC. Reversal of delta 9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav. 2002;71:333–340. doi: 10.1016/s0091-3057(01)00694-3. [DOI] [PubMed] [Google Scholar]
- 11.Ravinet Trillou C, Delgorge C, Menet C, Arnone M, Soubrie P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 12.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong WI, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartness TJ, Wade GN, Goldman BD. Are the short-photoperiod-induced decreases in serum prolactin responsible for the seasonal changes in energy balance in Syrian and Siberian hamsters? J Exp Zool. 1987;244:437–454. doi: 10.1002/jez.1402440310. [DOI] [PubMed] [Google Scholar]
- 15.Bartness TJ, Hamilton JM, Wade GN, Goldman BD. Regional differences in fat pad responses to short days in Siberian hamsters. Am J Physiol. 1989;257:R1533–R1540. doi: 10.1152/ajpregu.1989.257.6.R1533. [DOI] [PubMed] [Google Scholar]
- 16.Bartness TJ, Keen-Rhinehart E, Dailey MJ, Teubner BJ. Neural and hormonal control of food hoarding. Am J Physiol Regul Integr Comp Physiol. 2011;301:R641–R655. doi: 10.1152/ajpregu.00137.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goy RB, McEwan BS. Sexual Differentiation of the Brain. Cambridge, MA: MIT Press; 1980. [Google Scholar]
- 18.Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, et al. Transdermal nicotine alters some of marihuana’s effects in male and female volunteers. Drug Alcohol Depend. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291:203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- 21.Maccarrone M, Bari M, Di Rienzo M, Finazzi-Agro A, Rossi A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros. Evidence for a synergistic effect of leptin. J Biol Chem. 2003;278:32726–32732. doi: 10.1074/jbc.M302123200. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 23.Flint WE. Die Zwerghamster der Palaearktischen Fauna. Lutherstadt Wittenberg: Ziemsen Verlag; 1966. [Google Scholar]
- 24.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- 25.Durazzo A, Proud K, Demas GE. Experimentally induced sickness decreases food intake, but not hoarding, in Siberian hamsters (Phodopus sungorus) Behav Processes. 2008;79:195–198. doi: 10.1016/j.beproc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Berthoud HR. The caudal brainstem and the control of food intake and energy balance. In: Stricker EMW, editor. Neurobiology of Food and Fluid Intake. New York, NY: Springer US; 2004. pp. 195–240. [Google Scholar]
- 27.Grill HJ, Norgren R. Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 1978;201:267–269. doi: 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan CW, Christie MJ. Retrograde signalling by endocannabinoids. In: Pertwee RG, editor. Handbook of Experimental Pharamacology. Berlin: Springer; 2005. pp. 367–383. [DOI] [PubMed] [Google Scholar]
- 29.Jelsing J, Larsen PJ, Vrang N. The effect of leptin receptor deficiency and fasting on cannabinoid receptor 1 mRNA expression in the rat hypothalamus, brainstem and nodose ganglion. Neurosci Lett. 2009;463:125–129. doi: 10.1016/j.neulet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Pagano C, Rossato M, Vettor R. Endocannabinoids, adipose tissue and lipid metabolism. J Neuroendocrinol. 2008;20(Suppl. 1):124–129. doi: 10.1111/j.1365-2826.2008.01690.x. [DOI] [PubMed] [Google Scholar]
- 31.Annuzzi G, Piscitelli F, Di Marino L, Patti L, Giacco R, Costabile G, et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis. 2010;9:43. doi: 10.1186/1476-511X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verty AN, Allen AM, Oldfield BJ. The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity (Silver Spring) 2009;17:254–261. doi: 10.1038/oby.2008.509. [DOI] [PubMed] [Google Scholar]
- 33.Di Marzo V, Maccarrone M. FAAH and anandamide: is 2-AG really the odd one out? Trends Pharmacol Sci. 2008;29:229–233. doi: 10.1016/j.tips.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Helwig M, Archer ZA, Heldmaier G, Tups A, Mercer JG, Klingenspor M. Photoperiodic regulation of satiety mediating neuropeptides in the brainstem of the seasonal Siberian hamster (Phodopus sungorus) J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:631–642. doi: 10.1007/s00359-009-0438-3. [DOI] [PubMed] [Google Scholar]
- 35.Knopper LD, Boily P. The energy budget of captive Siberian hamsters, Phodopus sungorus, exposed to photoperiod changes: mass loss is caused by a voluntary decrease in food intake. Physiol Biochem Zool. 2000;73:517–522. doi: 10.1086/317730. [DOI] [PubMed] [Google Scholar]
- 36.Warner A, Jethwa PH, Wyse CA, I’Anson H, Brameld JM, Ebling FJ. Effects of photoperiod on daily locomotor activity, energy expenditure and feeding behavior in a seasonal mammal. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1409–R1416. doi: 10.1152/ajpregu.00279.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 38.Barrett P, Ross AW, Balik A, Littlewood PA, Mercer JG, Moar KM, et al. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinology. 2005;146:1930–1939. doi: 10.1210/en.2004-1452. [DOI] [PubMed] [Google Scholar]
- 39.Ross AW, Bell LM, Littlewood PA, Mercer JG, Barrett P, Morgan PJ. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinology. 2005;146:1940–1947. doi: 10.1210/en.2004-1538. [DOI] [PubMed] [Google Scholar]
- 40.Ross AW, Webster CA, Mercer JG, Moar KM, Ebling FJ, Schuhler S, et al. Photoperiodic regulation of hypothalamic retinoid signaling: association of retinoid X receptor gamma with body weight. Endocrinology. 2004;145:13–20. doi: 10.1210/en.2003-0838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.