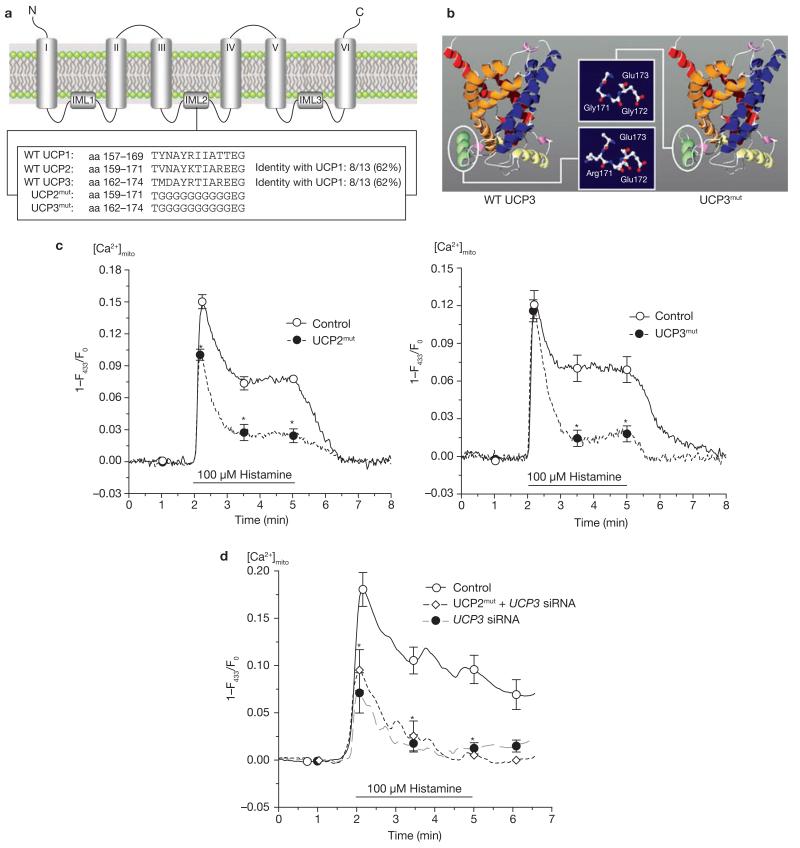
Figure 4. Mutagenesis of UCP2 and UCP3 resulted in loss of Ca2+ function of the proteins and provided dominant-negative UCP homologues.
(a) Sequence alignment and identification of specific domains common for UCP2 and UCP3, but not UCP1. Additional information is shown in the Supplementary Information. (b) Using the ROBETTA full-chain protein structure prediction server (http://robetta.bakerlab.org), the protein structure of UCP2 and UCP3 was predicted to form a channel-like structure. As indicated, the mutation was introduced in a helix that is located on the matrix side of the inner mitochondrial membrane and results in shortening of the helix loop and loss of polarity. (c) Functional consequences on mitochondrial Ca2+ uptake in single endothelial cells transiently expressing mitochondria-targeted ratiometric-pericam overexpressing mutated UCP2 (n = 25) or mutated UCP3 (n = 15). The asterisk indicates P <0.05 versus control (n = 17). (d) Mutated UCP2 failed to rescue mitochondrial Ca2+ uptake in single HeLa cells transfected with UCP3 siRNA. HeLa cells were transfected with either a non-functional control siRNA (n = 14), UCP3 siRNA (n = 9) or UCP3 siRNA together with mutated UCP2 (UCP2mut + UCP3 siRNA, n = 9). Asterisk indicates P <0.05 versus control siRNA.