Figure 2.
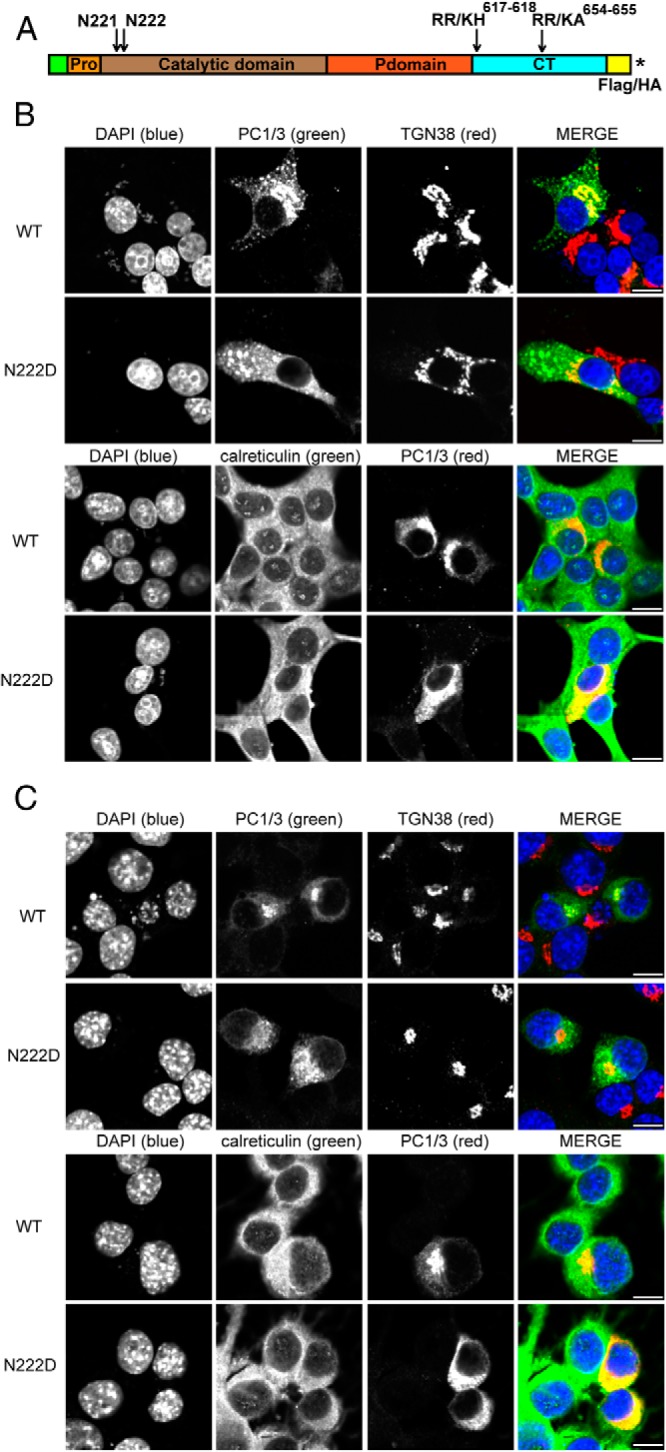
The N222D variant of PC1 is localized to the ER, whereas the WT protein is present in the Golgi and in the tips of endocrine cells. A, Schematic representation of PC1/3 showing the prodomain, catalytic domain, P domain, C-terminal domain, mutations incorporated, and the insertion positions of tags. An asterisk represents the stop codon. B, Rin5f cells were transiently transfected with PC1/3-WT-HA and PC1/3-N222D-HA constructs. Cells were fixed with 4% paraformaldehyde and processed as described (19). Colocalization was determined by immunostaining using a 1:100 dilution of monoclonal antibody to HA, a 1:100 dilution of sheep polyclonal antibody anti-TGN38, or a 1:50 dilution of goat polyclonal anti-calreticulin; these were followed by incubation with a 1:250 dilution of Cy2 (green)- or Cy3 (red)-conjugated antimouse immunoglobulin G, Cy2-conjugated antigoat, and Cy3-conjugated antisheep, respectively. C, In order to confirm that differences are due to the N222D mutation, Neuro2a cells were transiently transfected with PC1/3 constructs lacking the mutations that block carboxy-terminal processing. PC1/3-WT-Flag and PC1/3-N222D (untagged) were transfected and were treated as in B, except that PC1/3 was imaged using a rabbit polyclonal anti-PC1/3 antibody (2B5) together with either Cy2- or Cy3-conjugated antirabbit IgG. Scale bar, 10 μm.
