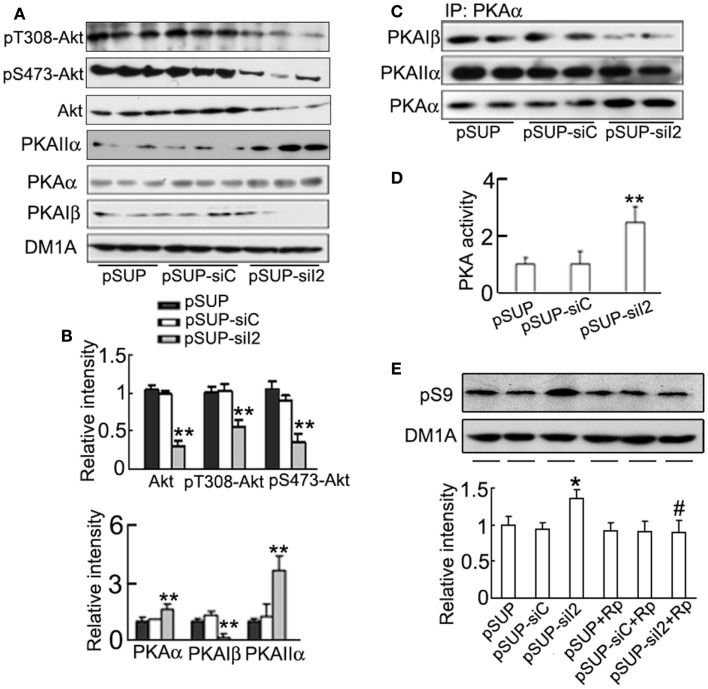
Figure 8.
Activation of PKA is responsible for the phosphorylation of GSK-3β at Ser9 induced by knockdown. HEK293/tau cells were transfected with (pSUP-siI2) for 24 h, and pSUP and pSUP-siC were expressed as the controls. (A,B) The levels of phosphorylated Akt at Thr308, Ser 473, total Akt, PKAα, PKAIIα, and PKAIβ were estimated by Western blotting (A) and quantitative analysis (B). (C) The cell lysates were subjected to immunoprecipitation (IP) with anti-PKAα antibody, and the precipitates were probed by anti-PKAα, anti-PKAIβ, or anti-PKAIIα. (D) The activity of PKA was also measured by a PKA assay kit as described in the Section “Materials and Methods.” (E) The cells with overexpression of pSUP-siI2, pSUP-siC, or the vector were treated with Rp-cAMPS (Rp, 10 μM) for 30 min, and then pS9-GSK-3β was detected by Western blotting and quantitative analysis. The relative intensity was normalized against DM1A and expressed by setting pSUP as 1. The data were presented as mean ± SD of three independent experiments. *p < 0.05;**p < 0.01 vs. pSUP; #p < 0.05 vs. pSUP-siI2.