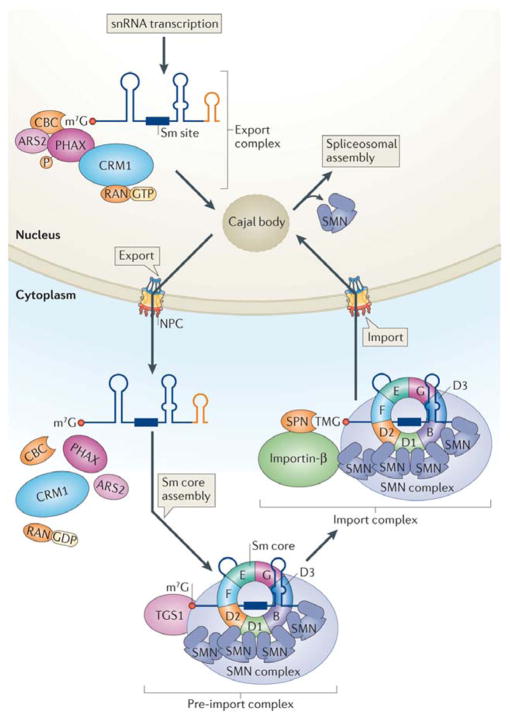
Figure 2. Maturation of snRNAs requires nuclear and cytoplasmic regulatory steps.
The snRNA pre-export complex consists of the heterodimeric cap-binding complex (CBC), arsenite resistance protein 2 (ARS2), the hyperphosphorylated form of the export adaptor PHAX and the large multi-subunit Integrator complex (not shown). Upon release from the site of snRNA transcription, the pre-export complex is remodelled within the nucleoplasm to form the export complex. This step involves removal of Integrator proteins and binding of the export receptor CRM1 (chromosome region maintenance 1) and the GTP-bound form of the RAN GTPase. Nucleoplasmic remodelling probably includes a Cajal body-mediated surveillance step to ensure RNP quality. Once transported to the cytoplasm, these export factors dissociate from the pre-snRNA prior to Sm core assembly and exonucleolytic trimming of the snRNA 3′ end (orange stem sloop). Following assembly of the Sm core snRNP (detailed in Fig. 3), the 7-methylguanosine (m7G) cap is hypermethylated by TGS1 (trimethylguanosine synthase 1) to form a 2,2,7-trimethylguanosine (TMG) cap. Generation of the TMG cap triggers assembly of the import complex, which includes the import adaptor snurportin (SPN) and the import receptor importin-β; both SPN and the SMN complex make functional contacts with importin-β (for simplicity, other components of the SMN complex are not depicted). Upon nuclear re-entry, the Sm snRNPs transiently localize to Cajal bodies for nuclear maturation steps, followed by dissociation from SMN and storage within splicing factor compartments called nuclear speckles. Spliceosome assembly (detailed in Fig. 4) takes place at sites of pre-mRNA transcription.