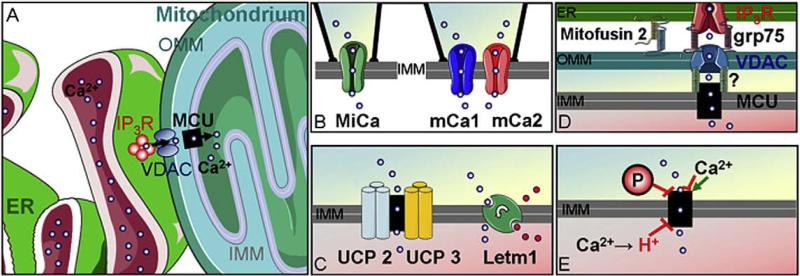
Fig. 1. Schematic illustration of mitochondrial Ca2+ uptake channels/carrier in the IMM, potential protagonists and the complexity if the mitochondrial environment.
(A) The complex environmental aspects of mitochondria in intact cells are illustrated. Local Ca2+ transfer from the ER via IP3 mediated Ca2+ release. VDAC as porines in the OMM deliver Ca2+ ions to the MCU or Ca2+ exchanger at the IMM. (B) Electrophysiological characterizations revealed distinct mitochondrial Ca2+ channels: the MiCa in COS-7 cells and mCa1 as well as mCa2 in human ventricular myocytes. (C) Overexpression and siRNA mediated knock-down of UCP2/3 suggest a fundamental importance of these proteins for mitochondrial Ca2+ uniport. A genome-wide RNAi screen identified Letm1 as a mitochondrial Ca2+/H+ antiporter that significantly contributes to mitochondrial Ca2+ uptake in the physiological range of cytosolic Ca2+ elevation. (D) ER-mitochondria contact sites are stabilized by mitofusin 2 and probably other, so far unknown, proteins. The chaperon grp75 was shown to link the IP3R to the VDAC in the OMM. Although proteins/factors that might link the VDAC to mitochondrial Ca2+ channels of the IMM have not been identified so far it is tempting to speculate that Ca2+ enters mitochondria via a Ca2+ tunnel spanning the OMM and IMM. (E) Evidence accumulated that kinases as well as Ca2+ and Ca2+-calmodulin differentially modulate the activities of mitochondrial Ca2+ channels.