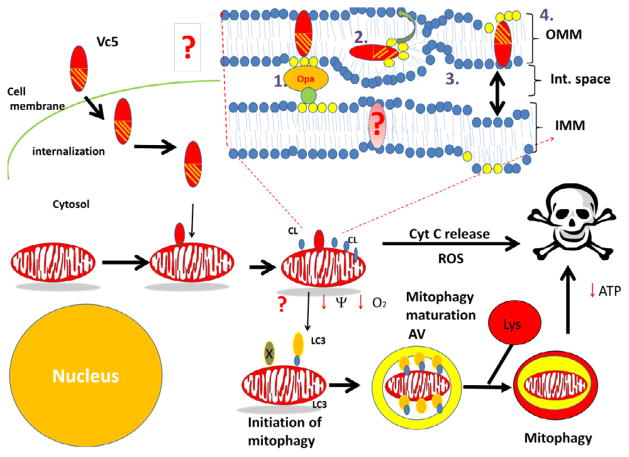
Figure 5.
Proposed figure model describing the interaction of cytotoxins with cell membranes, and its downstream effects on mitochondrial function and survival following its internalization. Cytotoxin on the extracellular space interacts with the cell membrane of the target cell. Cytotoxin is then internalized into the cytosol through uncharacterized mechanisms and can translocate to the mitochondrion. Upon binding to the OMM, cytotoxin induces a disorganization of the phospholipid bilayer which leads to the externalization of CL, rupture of the OMM by destroying the CL contact sites that keep the IMM in contact with the OMM and required for mitochondrial integrity (formed by the interaction of IMM-localized Opa1 and other proteins with CL), and can form transient inverted micelles that can be translocated to the IMM. At the IMM, cardiotoxin can also promote a disorganization of the lipid bilayer and disrupt oxidative phosphorylation as evidenced by a loss of ATP levels and a collapse of the transmembrane potential, and fission of adjacent IMM membranes. Fragmented mitochondria can either be sequestered by autophagosomes leading to their lysosomal-mediated hydrolysis or may release cytochrome C which initiates downstream apoptotic signaling by activating the apoptosome complex. The externalization of CL to the OMM triggers the removal of mitochondria via mitophagy by facilitating the association of the autophagy receptor microtubule-associated protein 1 light chain 3 (LC3) with externalized CL. The link between cardiotoxin-mediated cytotoxicity with mitophagy has not been established yet (shown as a question mark in this model). Hence, future studies are required to determine whether treating cells with cardiotoxins can promote mitophagy in cancer cells by disrupting the OMM structure and causing the externalization of CL.