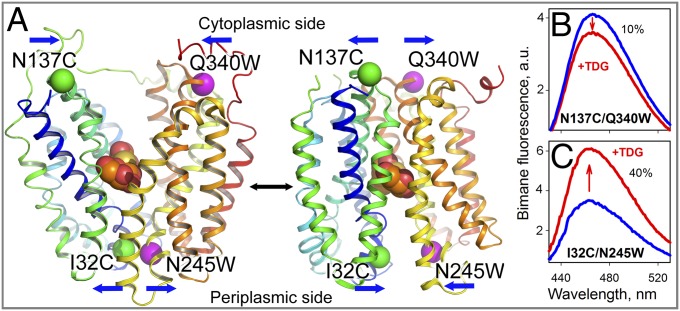
Fig. 1.
Detection of conformational changes in LacY by Trp quenching of bimane fluorescence. (A) Side view of LacY with cytoplasmic- or periplasmic-open cavities [Protein Data Bank (PDB) ID code 2CFQ (Left) and PDB ID code 4OAA (Right)]. Transmembrane helices are rainbow-colored from blue (helix I) to red (helix XII), and a bound galactoside (orange spheres) is shown at the apex of the cavities. Pairs of Cys-Trp replacements introduced individually on opposite sides of the periplasmic or cytoplasmic cavities are shown with the indicated positions of Cα atoms of Trp (pink spheres) and PDT-bimane–labeled Cys (green spheres) residues. Blue arrows show the direction of movement. (B) Quenching of bimane fluorescence on the cytoplasmic side of LacY observed with bimane-labeled N137C/Q340W mutant. a.u., arbitrary units. (C) Unquenching of bimane fluorescence on the periplasmic side of LacY observed with bimane-labeled I32C/N245W mutant. Blue and red lines represent bimane emission spectra before and after addition of 10 mM TDG, respectively. Spectra were recorded with excitation at 380 nm with 0.3 μM protein in 50 mM NaPi/0.02% DDM (pH 7.5).