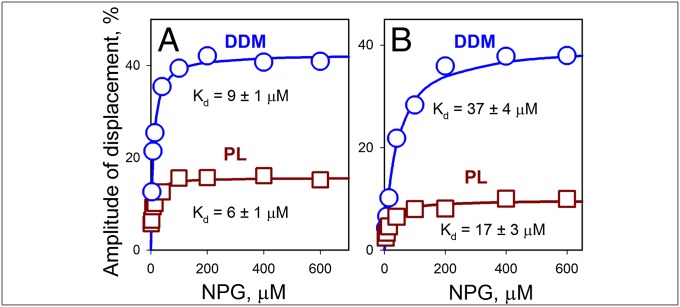
Fig. 2.
Sugar binding to bimane-labeled LacY mutants. NPG-binding affinity was measured with periplasmic mutant I32C/N245W (A) and cytoplasmic mutant N137C/Q340W (B) in displacement experiments with proteins dissolved in DDM (○) or reconstituted into proteoliposomes (PL; □). Stopped-flow traces were recorded after mixing a saturating concentration of TDG (15 mM) with protein preincubated with the indicated concentrations of NPG (Fig. S5). The amplitude of Trp fluorescence change is expressed as a percentage of the final fluorescence level of the individual stopped-flow trace. Kd values estimated from hyperbolic fits of concentration dependencies of displacement amplitudes are given. Average values of koff calculated with mutant I32C/N245W from individual experiments at nine NPG concentrations are 17 ± 1 s−1 and 25 ± 2 s−1 in DDM and PL, respectively. For mutant N137C/Q340W, koff values calculated from experiments at eight NPG concentrations are 50 ± 3 s−1 and 69 ± 15 s−1 in DDM and PL, respectively.