Abstract
Purpose
Malignant peripheral nerve sheath tumor (MPNST) is a highly aggressive tumor type that is resistant to chemotherapy and there are no effective therapies. MPNSTs have been shown to have gene amplification for receptor tyrosine kinases (RTKs), PDGFR and c-Kit. We tested the c-Kit inhibitor, imatinib, and PLX3397, a selective c-Fms and c-Kit inhibitor, to evaluate their efficacy against MPNST cells in vitro and in vivo.
Experimental Design
We tested the efficacy of imatinib or PLX3397 either alone or in combination with TORC1 inhibitor rapamycin in a cell proliferation assay in vitro and by immunoblotting to determine target inhibition. Immunoblotting and immunohistochemical analysis was further carried out using xenograft samples in vivo.
Results
Our in vitro studies show that imatinib and PLX3397 similarly inhibit cell growth and this can be enhanced with rapamycin with comparable target specificity. However, in vivo studies clearly demonstrate that compared to imatinib, PLX3397 results in sustained blockade of c-Kit, c-Fms and PDGFRβ, resulting in significant suppression of tumor growth. Moreover, staining for Iba-1, a marker for macrophages, indicates that PLX3397 results in significant depletion of macrophages in the growing tumors. The combination of PLX3397 and rapamycin results in even greater macrophage depletion with continued growth suppression, even when the drug treatment is discontinued.
Conclusions
Taken together, our data strongly suggests that PLX3397 is superior to imatinib in the treatment of MPNST, and the combination of PLX3397 with a TORC1 inhibitor could provide a new therapeutic approach for the treatment of this disease.
Keywords: PLX3397, rapamycin, mTOR, Fms-Kit
Introduction
Malignant peripheral nerve sheath tumors (MPNST) are soft-tissue tumors with a very poor prognosis (1). They are highly aggressive and therapeutically resistant tumors that arise in connective tissue surrounding peripheral nerves. MPNSTs occur in a subset of patients with Neurofibromatosis type 1 (NF1), an autosomal dominant genetic disorder (2, 3). There is a 10% chance that patients with NF1 will develop MPNSTs in their lifetime (4, 5). Despite advances in cancer treatment, MPNSTs typically remain fatal and there is an unmet need to develop new therapeutic strategies in this disease setting.
Growth factor dependent pathways driven specifically by receptor tyrosine kinases (RTKs) have been of particular interest due to their crucial role in tumor progression and survival. Targeting single or multiple RTK pathways using small molecule inhibitors is an attractive chemotherapeutic treatment option for blocking sarcoma cell growth. Such inhibitors have been used in sarcoma patients with some promising results (6, 7). In addition to the RTKs, mTOR (mammalian target of Rapamycin) protein plays a key role in AKT activation and downstream survival signaling. Blocking RTK signaling pathways such as c-Kit and PDGFR using multi-targeted inhibitors like imatinib has been used with some positive results in vitro in MPNSTs (8–10). However, MPNSTs still remain one of the most challenging sarcoma subtypes to treat and novel therapeutic approaches are urgently needed to treat this disease.
MPNSTs have been shown to have gene amplification for receptor tyrosine kinases such as PDGFR as well as c-Kit (9, 11). The role of c-Kit oncogenic mutations in gastro-intestinal stromal tumors (GIST) is well established (12, 13). PDGFRα has also been shown to be amplified in tumors and in cell lines from patients with MPNST. c-Kit was similarly amplified in four of the five tumors and patient derived cell lines. MPNSTs can also carry PDGFRα mutations. No mutations in c-Kit have been reported. Imatinib mesylate (Imatinib), an inhibitor of c-Kit and PDGFR, which is approved for the treatment of GIST, has been shown to be active in patients with plexiform neurofibromas, a slow-growing, chemotherapy resistant tumor that develops in patients with NF (14). However, in clinical trials, response rates to imatinib in neurofibromatosis are only in the order of 17%, indicating that alternate signaling pathways must be involved in the growth and development of tumors associated with NF1 loss (14). Though imatinib has never been formally tested in MPNST, it has been evaluated in patients affected by NF1 loss who developed GIST. Similar to MPNST patients, the overall prognosis of this patient population is poor, and the response to imatinib in NF1-associated GIST is very low (15).
In this current study, we tested the multi-targeted tyrosine kinase inhibitor, PLX3397. PLX3397 selectively inhibits c-Fms and c-Kit receptor tyrosine kinases with biochemical IC50 values of 0.02μM and 0.01μM respectively (16). Recently it has been reported that macrophage infiltration of both mouse and human neurofibromas correlates with disease progression. Macrophages account for almost half of neurofibroma cells. In the Dhh-Cre/Nf1 mouse model of neurofibroma, PLX3397 has been shown to cause neurofibroma regressions and to block macrophage infiltration (17). In this study, we elected to compare imatinib to PLX3397 against MPNST cells. Our studies indicate that PLX3397 is a superior in vivo kinase inhibitor and results in significantly greater suppression of tumor volume and macrophage depletion. Furthermore, inhibition of mTOR signaling, which is also active in this disease, with rapamycin further enhances this effect. Findings from our studies suggest that PLX3397 in combination with an mTOR inhibitor is biologically active in MPNST and should be explored for future clinical development in patients with MPNSTs.
Materials and Methods
Drugs
PLX3397 (Fms/Kit inhibitor) and vehicle carrier, were provided by Plexxikon Inc. (Berkeley, CA) in the form of powder for in vitro studies, or chow containing PLX3397 or control chow, respectively, for in vivo studies. Imatinib and Rapamycin were purchased from LC Laboratories (Woburn, MA).
Cell lines
MPNST and ST8814 cell lines have described previously (18). GIST882 cell line has also been described previously (19). Briefly, MPNST was derived from a patient with a high-grade peripheral nerve sheath tumor of the thigh. ST8814 was derived from a patient with NF1-associated MPNST of the thigh. Dr. Jonathan Fletcher, Dana Farber Cancer Institute, graciously supplied both these cell lines. In ST8814, a nonsense mutation (C910T) in codon 304 of exon 7 of NF1 has been reported (Y. Kloog, Tel Aviv University, Israel, personal communication to Dr. Schwartz). This is a pathogenic mutation leading to exon 7 skipping. These cell lines have been authenticated using Short Tandem Repeat DNA fingerprinting (20, 21). MPNST cells were able to form tumors when injected in nude mice; however, we were unable to grow ST8814 tumors in mice. All the three cell lines were maintained in RPMI-1640 media containing 10% FBS (HyClone, Thermo Scientific). For serum starvation, cells were plated in RPMI-1640 media without FBS overnight and then treated with the drugs in RPMI-1640 media containing 10% FBS for the indicated time.
Cell viability assays
Cell viability assays were carried out with the Dojindo Molecular Technologies Kit per manufacturer’s instructions. Briefly, 2,000 cells were plated in 96-well plates in RPMI media with 10% FBS and then treated with the indicated drugs the next day. Media was replaced with 100μL of media with 10% serum and 10% CCK-8 solution (Dojindo Molecular Technologies Kit). After 1 hour, the optical density was read at 450 nm using a Spectra Max 340 PC (Molecular Devices Corp.) to determine viability. Background values from negative control wells without cells were subtracted for final sample quantification. Data was plotted as % cell viability compared to DMSO (No Drug) control.
Western blots
Western blots were carried out as previously described (18). Briefly, cell lysates were prepared by washing the cells once with sterile PBS followed by scraping in radioimmunoprecipitation assay (RIPA) lysis buffer. For xenograft tissues, lysates were prepared by cutting the snap frozen tumor tissue into a small piece and then grinding it in RIPA lysis buffer using sample grinding kit (GE Healthcare). Protein concentrations were measured using Bio-Rad protein assay dye (Bio-Rad) and equal amounts of protein (20 or 30μg) were loaded on 4% to 12% gradient gels (Invitrogen) and transferred to PVDF membrane (Immobilon, Millipore) or nitrocellulose membrane (0.45 micron, Thermo Scientific) for the detection of phosphorylated c-Kit. After blocking with 5% milk, membranes were probed with primary antibodies overnight. Bound antibodies were detected with horseradish peroxidase secondary antibodies (GE Healthcare) and visualized by enhanced chemiluminescence reagent (GE Healthcare).
Xenograft Studies
Briefly, MPNST xenografts were transplanted subcutaneously in the flank of ICR/SCID mice. Once tumors reached a volume of 80–100mm3, the mice were randomized into different groups of 7–10 animals each and treated with the indicated drugs or vehicle control. Imatinib was used at a concentration of 30mg/kg i.p. QD, 5 days a week and rapamycin was used at a concentration of 20mg/kg i.p. on Monday, Wednesday and Friday. For studies involving PLX3397, mice were fed a diet with either control chow or PLX3397 chow for 3 weeks. Tumor size was measured twice weekly by caliper. The average tumor volume in each group was expressed in cubic millimeter and calculated using the formula p/6 × (large diameter) × (small diameter)2. Approximately 1–2 animals in each group were sacrificed after 3 weeks of drug treatment and the resected tumors were divided for formalin fixation (for hematoxylin/eosin staining and IHC) and snap frozen tissue (for western blot). Experiments were carried out under an Institutional Animal Care and Use Committee–approved protocol, and institutional guidelines for the proper and humane use of animals were followed.
Analysis of tumor associated macrophages (TAMs)
TAMs were isolated from control and drug treated xenograft samples (n=3 animals per group) as described previously (22). Briefly, tumor cells were isolated by mincing the tumor in collagenase/DNAase in Hank’s balanced salt solution (HBSS) followed by flow cytometric analysis using indicated antibodies. Data was plotted using Graph Pad Prism software with relevant population percentages plotted with ANOVA and t-test performed and indicated where appropriate.
Iba-1 and Ki67 staining and quantitation
Preparation of tissue sections for IHC and staining for Iba-1 and Ki67 was carried out by MSKCC molecular cytology core facility. The immunohistochemistry detection of Iba1 and Ki67 was performed using Discovery XT processor (Ventana Medical Systems). Staining and quantitation details are included in the supplemental methods.
Biostatistics
In vitro experiments were carried out at least 3 times unless otherwise indicated. Error bars shown in the graphs represent standard deviation. All the graphs were plotted using GraphPad Prism software. Statistical analyses were carried out using student’s t-test with 95% CI.
Results
PLX3397 treatment down-regulates receptor tyrosine kinase (RTK) phosphorylation and inhibits cell proliferation in MPNST and gastro-intestinal stromal tumor (GIST) cells
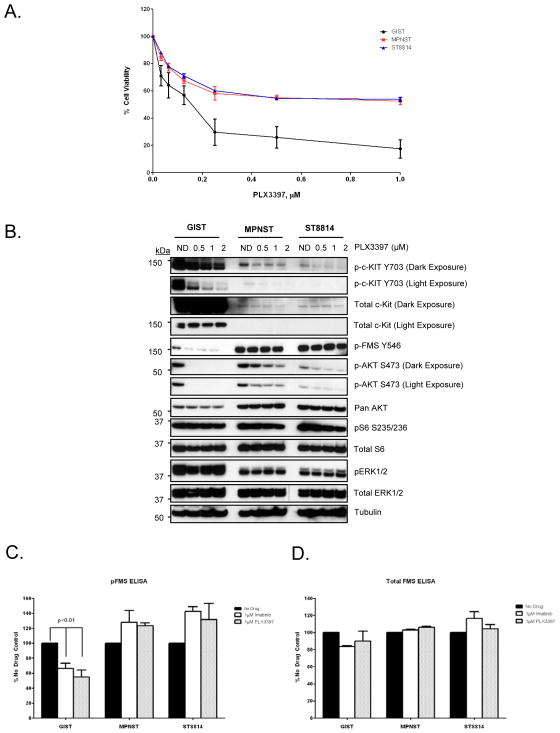
PLX3397 is a selective inhibitor of c-Fms and c-Kit tyrosine kinases with in vitro IC50 values of 0.02μM and 0.01μM respectively. Out of a panel of 226 different kinases including all the protein kinase subfamilies and lipid kinases, PLX3397 inhibited only five other kinases with low IC50 values (16). Since PLX3397 is a highly selective c-Fms/c-Kit inhibitor, we tested eight sarcoma cell lines to check for basal expression levels of c-Fms and c-Kit along with a GIST cell line, GIST882, as a positive control for c-Kit expression. Detectable levels of c-Kit expression were observed only in the two MPNST cell lines, MPNST and ST8814, in addition to GIST882 (Supplemental Fig. 1). However, c-Fms expression levels were detectable in all the cell lines tested. GIST882 had the highest levels of c-Kit (Supplemental Fig. 1). Based on this finding, we carried out an in vitro cell proliferation assay using different doses of PLX3397 in the two MPNST cell lines, MPNST and ST8814 as well as in the GIST cell line, GIST882. As seen in Fig. 1A, both MPNST cell lines and GIST882 cell line were susceptible to inhibition of proliferation by PLX3397. The IC50 value for the GIST cell line was approximately 0.1μM, whereas, for the MPNST cell lines, IC50 values were approximately between 0.5μM-1 μM. The GIST cells, which harbor a c-Kit mutation and have a higher basal expression of p-Kit, were more susceptible to growth inhibition by PLX3397 than the MPNST cells. Western blot analysis using various concentrations of PLX3397 confirmed that p-Kit, a known target of the drug, was inhibited in all the three cell lines tested (Fig. 1B). In addition to p-Kit inhibition, AKT phosphorylation (p-AKTS473) was also inhibited (Fig. 1B) with increasing drug concentration suggesting a role for c-Kit in inducing AKT phosphorylation in these cell lines. Tyrosine phosphorylation of c-Fms (Y546), another target of PLX3397, was inhibited only in the GIST cell line but not in MPNST cell lines. GIST cells showed higher basal levels of p-Kit (Y703), whereas, MPNST cell lines showed higher basal levels of p-Fms (Y546) (Fig. 1B).
Figure 1. Effects of PLX3397 in GIST, MPNST and ST8814.
A. GIST, MPNST and ST8814 cells were plated in 96-well plates and treated in triplicates with increasing doses of PLX3397 for 144 hours. Cell viability was measured using Dojindo Cell Counting Kit 8. B. The cells were grown to 60% confluency in 60-mm plates and treated for 24 hours with DMSO control or increasing concentrations of PLX3397 (0.5, 1 and 2 μmol/L). 30μg of RIPA lysates were loaded on SDS/PAGE and immunoblotted using indicated antibodies. C and D. Cells were grown to 60% confluency in 100-mm plates and treated for 24 hours with DMSO control, 1 μM Imatinib or 1 μM PLX3397. Protein lysates were then extracted and used for p-Fms and total Fms sandwich ELISA following the manufacturer’s protocol for DuoSet® IC ELISA kit by R&D Systems.
Phosphorylated c-Fms kinase is not down-regulated in MPNST cells in vitro
As shown in Fig. 1B, phosphorylation of c-Fms at Tyr546 was not down-regulated in MPNST cells compared to GIST cells. In order to test whether PLX3397 can down-regulate tyrosine phosphorylated c-Fms in MPNST cells, we carried out an in vitro ELISA assay to test the efficacy of PLX3397 in blocking overall c-Fms tyrosine phosphorylation instead of detecting phosphorylation at Tyr546 only. Imatinib has been shown to inhibit c-Fms at therapeutic concentrations (23, 24). Our ELISA results showed that tyrosine phosphorylation of c-Fms is inhibited neither by imatinib nor by PLX3397 in MPNST cells (Fig. 1C). However, in GIST cells, both imatinib and PLX3397 inhibited c-Fms tyrosine phosphorylation by 40–50% when compared to no drug control (p<0.01) (Fig. 1C). This result is consistent with the results obtained using western blot analysis (Fig. 1B). Total levels of c-Fms remained unchanged after the drug treatment in all the cell lines tested (Fig. 1D).
PLX3397 and Imatinib inhibit MPNST cell proliferation and down-regulate downstream targets effectively in vitro
Since PLX3397 has recently been shown to impair tumor maintenance in mouse models of MPNSTs (17), we decided to focus our subsequent in vitro and in vivo experiments in the two MPNST cell lines.
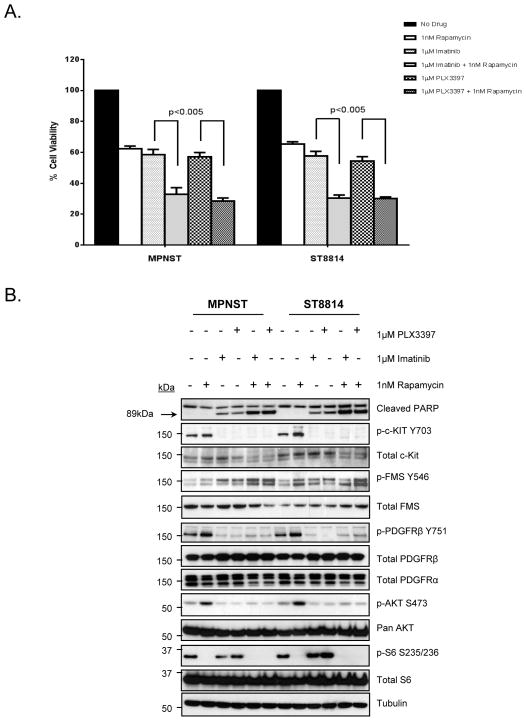
RTK/PI3K/AKT/mTOR pathways have been shown to be critical for oncogenic growth and survival in soft tissue sarcomas. TORC1 downstream effectors including S6 kinase (S6K) and S6 ribosomal protein (S6), a 40S ribosomal component, are critical in regulation of protein translation and cell proliferation (25). mTOR signaling has been shown to be particularly important for MPNST growth. However, mTOR inhibitors have shown minimal activity as single agents in sarcoma clinical studies (26, 27). PLX3397 does not inhibit TORC1 downstream targets including S6K and S6 ribosomal protein (S6) (Fig. 1B). Therefore, we elected to test in the MPNST cells whether the combination of PLX3397 with the TORC1 inhibitor, rapamycin, could more effectively inhibit cell proliferation and to assess how this affected AKT and mTOR signaling. Results from the in vitro cell proliferation assay (Fig. 2A) show that either PLX3397 or imatinib, when combined with rapamycin, were more efficient in inhibiting MPNST cell proliferation than any single agent drug treatment. Single agent treatment resulted in approximately 40–45% decrease in cell viability compared to no drug control, whereas, combination therapy resulted in approximately 70% inhibition of cell proliferation (Fig. 2A) (p<0.005). Western blot analysis confirmed inhibition of p-Kit (Y703) and p-PDGFRβ (Y751) with both single agent and combination therapy (Fig. 2B). Treatment with PLX3397 or imatinib alone did not inhibit p-S6 and treatment with rapamycin alone did not inhibit either p-Kit or p-PDGFRβ. Rapamycin alone was able to activate p-Kit, p-PDGFRβ, and p-AKT presumably through the release of negative feedback mechanisms (28). However, the combinations were effective in inhibiting phosphorylation of both RTKs as well as phosphorylation of AKT and S6. Similar to earlier results (Fig. 1B and Fig. 1C), p-Fms was not inhibited in MPNST cell lines with any of the drug treatments. Induction of apoptosis was confirmed by PARP cleavage upon treatment with either PLX3397 or imatinib (Fig. 2B, top panel) but it was more pronounced when either drug was combined with rapamycin. To test whether c-Kit or PDGFRβ or both play a role in inhibiting cell proliferation in vitro, we carried out siRNA mediated knockdown of both the RTKs either alone or in combination. PDGFRβ knockdown resulted in approximately 50% inhibition, whereas, c-Kit knockdown resulted in a modest 30% inhibition of cell proliferation (Supplemental Fig. 2) suggesting a greater role of PDGFRβ in cell proliferation. However, combined knockdown of both c-Kit and PDGFRβ resulted in approximately 70% inhibition of cell proliferation (Supplemental Fig. 2). PDGFRβ has not been evaluated as a target for PLX3397. To test whether PDGFRβ is a direct target of the drug, we carried out ligand stimulation experiment and tested for inhibition of p-PDGFRβ in MPNST and ST8814 cell lines. As expected, no p-PDGFRβ signal was detected under no serum conditions, but when cells were stimulated with PDGFB/B, p-PDGFRβ was inhibited only in cells treated with PLX3397 but not with rapamycin alone (Supplemental Fig. 3). Many of the small molecular inhibitors that block RTK pathways such as sorafenib and lapatinib have been considered as cytostatic agents rather than cytotoxic drugs (29). Fig. 1A and 2A suggest that the effects of PLX3397 appear to be cytostatic rather than cytotoxic. To test this, we carried out flow cytometric cell cycle analysis after PLX3397 treatment either as single agent or in combination with rapamycin in MPNST and ST8814 cell lines. Treatment with PLX3397 but not rapamycin alone induced a G1-cell cycle arrest (Supplemental Fig. 4A), which was more pronounced when the two drugs were combined. Cyclin D1 down regulation has been shown to play a role in G1- cell cycle arrest in other cancer cell lines (30). Western blot analysis after treatment with imatinib or PLX3397 alone induced significant down-regulation of cyclin D1 and a significant decrease in hyper-phosphorylated form of retinoblastoma protein (p-Rb) (Supplemental Fig. 4B). Total levels of Rb and p53 remained unchanged.
Figure 2. Comparison of Imatinib versus PLX3397 in MPNST and ST8814 cell lines.
A. MPNST and ST8814 cells were plated in 96-well plates and treated in five wells per condition with 1 μmol/L of either Imatinib or PLX3397 with or without 1nM Rapamycin for 144 hours. Cell viability was measured using Dojindo Cell Counting Kit 8. B. Cells were grown to 60% confluency in 100-mm plates in serum starvation media and treated for another 24 hours in 10%FBS media using indicated drugs. 30μg of RIPA lysates were then loaded on SDS/PAGE and immunoblotted using indicated antibodies.
PLX3397 treatment results in sustained inhibition of RTKs and inhibits macrophage activation by blocking c-Fms
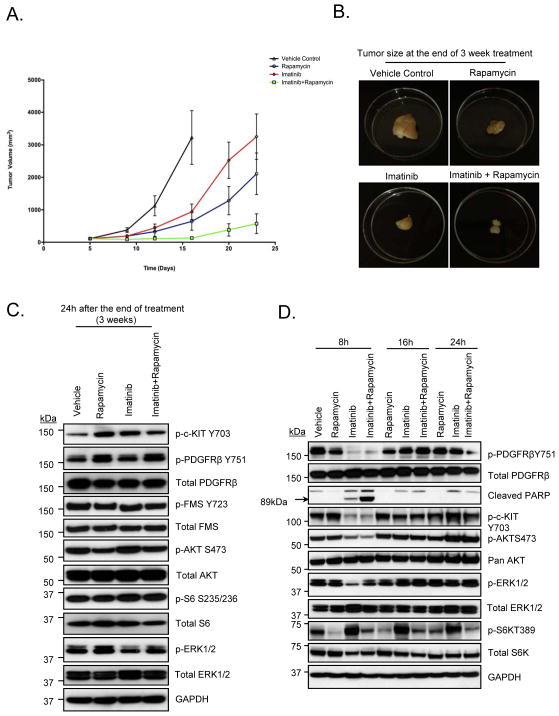
Imatinib and PLX3397 both have overlapping target specificity in vitro and were similarly effective in blocking in vitro cell proliferation in MPNST cells (Fig. 2A). Therefore, we carried out mouse xenograft experiments to test whether the in vivo target inhibition profile of the two drugs was also similar. As shown in Fig. 3A and 3B, at the end of 3 week treatment, imatinib and rapamycin when used in combination resulted in enhanced suppression of tumor volume compared to either of the drugs alone (p<0.005). However, at completion of treatment (week 3) when xenograft lysates were analyzed by western blot, none of the target tyrosine kinases or TORC1 effectors (S6) were effectively down-regulated (Fig. 3C). Since the animals were sacrificed approximately 24 hours after the last drug treatment, we hypothesized that the signaling pathways were re-activated in the 24 hours after the treatment. To test this hypothesis, we carried out a time course experiment in MPNST xenografts, wherein; the animals were sacrificed 8, 16 or 24 hours after the treatment on Day 1. As shown in Fig. 3D, imatinib alone inhibited p-Kit and p-PDGFRβ but not p-S6. Phosphorylation of S6K, a known mTORC1 target, either alone or in combination with imatinib, was down-regulated effectively confirming that the mTOR pathway is down-regulated by rapamycin in vivo. After 8 hours of treatment, p-Kit, p-PDGFRβ, p-AKT, and p-S6K were only inhibited in mice treated with imatinib in combination with rapamycin (Fig. 3D). A stronger signal for PARP cleavage was seen with the combination of imatinib and rapamycin as compared to imatinib alone (Fig. 3D). After 16 hours, p-Kit, p-PDGFRβ and p-AKT were re-activated even in animals treated with the imatinib and rapamycin combination suggesting that the signaling pathways are only transiently inhibited after this drug treatment (Fig. 3D).
Figure 3. Effect of Imatinib and/or Rapamycin in MPNST xenografts.
A and B. Tumor growth of MPNST xenografts treated with the indicated drugs for a period of 3 weeks is shown. See methods for drug dosing schedule. C and D. 30μg of RIPA lysates obtained using sample grinding kit (GE healthcare) from xenograft tissues at the end of 3-week treatment or at various time points post-treatment were loaded on SDS/PAGE and immunoblotted using indicated antibodies.
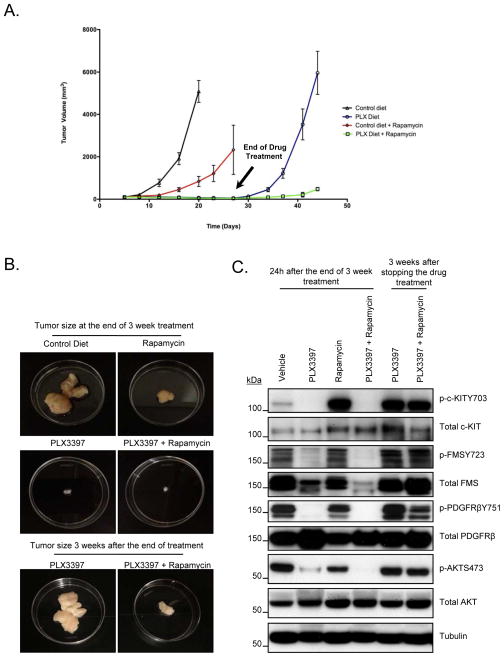
Next, we elected to repeat the mouse xenograft experiments using PLX3397. As shown (Fig. 4A and 4B), PLX3397 treatment resulted in significant tumor growth suppression when used either alone or in combination with rapamycin (p<0.005). As long as the animals were treated with PLX3397, the tumor was maintained at a significantly lower volume than vehicle control or rapamycin alone (Fig. 4A and 4B). We did not observe any significant difference between PLX3397 alone versus PLX3397 and rapamycin combination in terms of tumor volume suppression (Fig. 4A and 4B). Therefore, we decided to test whether there was a difference between the tumor re-growth when the drug treatments were discontinued. The drug treatment was discontinued after 3 weeks, and the mice were monitored for increase in tumor volume for an additional 3 weeks. Surprisingly, tumors treated with PLX3397 alone grew back rapidly, whereas, mice treated with PLX3397 and rapamycin in combination showed a slower increase in tumor re-growth (Fig. 4A and 4B). Western blot analysis was carried out to determine whether PLX3397 inhibited p-Kit and p-PDGFRβ. Similar to imatinib treatment, the animals were sacrificed approximately 24 hours after the completion of treatment on week 3. However, unlike imatinib, PLX3397 was able to achieve sustained down regulation of p-Kit and p-PDGFRβ signaling, even after 24 hours after the last drug treatment. Furthermore, in contrast to the effect of PLX3397 in vitro, p-Fms was significantly inhibited in vivo (Fig. 4C). We were unable to detect consistent expression levels of total S6 protein in these xenograft lysates; therefore, phosphorylation levels of S6 could not be determined. Nevertheless, p-AKT was inhibited significantly when animals were treated with PLX3397 alone and p-AKT was further down-regulated when PLX3397 was used in combination with rapamycin (Fig. 4C). Ki67 staining indicated a greater inhibition of cell proliferation with PLX3397 than with rapamycin (Supplemental Fig. 5A and B). Treatment with PLX3397 and rapamycin resulted in even higher inhibition of cellular proliferation than when treated with PLX3397 alone (p<0.005). Xenograft lysates obtained from animals sacrificed 3 weeks after discontinuing the drug treatment showed that all the signaling pathways were re-activated when the drug treatments were discontinued (Fig. 4C, lanes 5 and 6). Staining for CD31, a known endothelial cell marker, did not show any significant change after PLX3397 treatment compared to control group (Supplemental Fig. 5C) As reported previously (31) rapamycin treatment alone caused a reduction in CD31 signal indicating reduced tumor vasculature (Supplemental Fig. 5C).
Figure 4. Effect of PLX3397 and/or Rapamycin treatment in MPNST xenografts.
A and B. Tumor growth of MPNST xenografts treated with the indicated drugs is shown. Drug treatment was stopped on Day 17 for Control Diet group (since the tumors were huge) and on Day 24 for the remaining 3 treatment groups. Tumor growth measurements were continued for PLX Diet and PLX Diet + Rapamycin groups for another 3 weeks after stopping the drug treatment. C. 30μg of RIPA lysates obtained using sample grinding kit (GE healthcare) from xenograft tissues at the end of 3 week treatment and 3 weeks after the drug treatment was stopped were loaded on SDS/PAGE and immunoblotted using indicated antibodies.
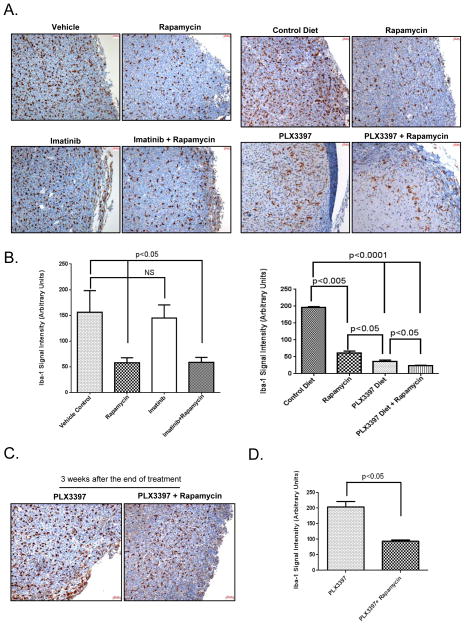
Ionized calcium-binding adapter molecule 1 or Iba-1 is a protein expressed specifically by macrophage/microglia cells and is involved tumor growth and maintenance mediated through c-Fms kinase (32, 33). To test whether macrophage population was affected by imatinib and PLX3397 drug treatment, we carried out immunohistochemical staining to detect Iba-1. As shown in Fig. 5A and B, imatinib treatment alone did not affect Iba-1 signal as compared to vehicle control (p=0.365, not significant). However, Iba-1 signal was decreased significantly compared to vehicle control, when mice were treated with rapamycin either as a single agent or in combination with imatinib (p<0.05, Fig. 5A and B).
Figure 5. Effect of Imatinib and PLX3397 and/or Rapamycin on Iba-1.
A. Xenograft tissues obtained from mice at the end of 3-week treatment with the indicated drugs were stained immuno-histochemically using Iba-1 antibody. Scale bar (50μm) is shown in the right hand corner of each image. Images from a tumor obtained at the end of 3 week treatment from a representative experiment reproduced at lease three independent times are shown. B. Iba-1 signal (brown staining) was quantitated using MetaMorph image analysis software (Molecular Devices). Iba-1 signal was quantified from at least 3 different high power fields and plotted as arbitrary intensity units. (NS=Not Significant). Quantitation of Iba-1 signal from a tumor obtained from a representative experiment reproduced at lease three independent times is shown. C. Xenograft tissues obtained at the end of 1 week after stopping the drug treatment were stained immuno-histochemically using Iba-1 antibody. Scale bar (50μm) is shown in the right hand corner of each image. Images of a tumor obtained from a representative experiment reproduced at lease two independent times are shown. D. Iba-1 signal (brown staining) was quantitated using MetaMorph image analysis software (Molecular Devices). Iba-1 signal was quantified from at least 3 different high power fields and plotted as arbitrary intensity units. Quantitation of Iba-1 signal from a tumor obtained from a representative experiment reproduced at lease two independent times is shown.
Similar to previously published reports (17, 34), PLX3397 significantly decreased macrophage population measured as Iba-1 signal (Fig. 5A and B, p<0.0001). In contrast to imatinib, the effect of rapamycin and PLX3397, was greater than either agent alone (p<0.05). Next, we determined Iba-1 signal in tumor samples obtained 3 weeks after stopping the drug treatment. As expected, Iba-1 signal increased (Fig. 5A and B versus 5C) when PLX3397 treatment was discontinued. However, when compared to PLX3397 alone after discontinuation, Iba-1 signal remained significantly suppressed at least for the first one week with the combination therapy of PLX3397 and rapamycin (Fig. 5Cand D ).
Macrophage depletion alone is not sufficient to suppress tumor growth in MPNST xenografts
To test whether the effects of PLX3397 on tumor growth suppression are mediated by inhibition of RTKs and/or macrophage depletion, we carried out xenograft studies by treating the animals using c-Fms specific drug (PLX5622) or a c-Fms specific antibody (AFS98) either alone or in combination with rapamycin and compared it against PLX3397. As observed earlier, PLX3397 either alone or in combination with rapamycin suppressed tumor growth significantly (Supplemental Figure 6A). Western blot analysis showed down-regulation of target RTKs including p-Kit, p-PDGFRβ and p-Fms (Supplemental Fig. 6B). PLX5622, a c-Fms specific drug, showed moderate down-regulation of p-Fms (Y723) on western blot. Modest to no down-regulation of other pathways including p-Kit, p-PDGFRβ and p-AKT was seen compared to PLX3397 (Supplemental Fig. 6B). As expected, c-Fms blockade by PLX5622 treatment resulted in macrophage depletion detected as reduced Iba-1 signal (Supplemental Fig. 6C). AFS98, a c-Fms specific antibody, treatment also showed moderate p-Fms down-regulation (Supplemental Fig. 7A) and as expected caused macrophage depletion detected as decreased percentage of live cells (Supplemental Fig. 7B). Though c-Fms blockade resulted in macrophage depletion (not comparable to PLX3397 however), it appeared that macrophage depletion alone was not sufficient to suppress tumor growth when xenografts were treated with PLX5622 or AFS98 alone (Supplemental Fig. 6A and 7C).
Combination with rapamycin further enhanced macrophage depletion (Supplemental Fig. 6C and 7B) and also resulted in better tumor suppression (Supplemental Fig. 6A and 7C). Flow cytometric analysis showed that most of the tumor associated macrophage population (TAMs) was M2-like and rapamycin treatment alone caused M2-like TAMS to shift to more M1-like population (Supplemental Fig. 8). Such an effect of mTOR inhibition by rapamycin on macrophage polarization has been reported previously (35). Sufficient numbers of macrophages could not be isolated from other treatment groups to carry out flow cytometric M1-like versus M2-like sorting.
Discussion
Malignant peripheral nerve sheath tumors (MPNSTs) that arise in patients with type 1 neurofibromatosis (NF1) are classically associated with activation of the Ras pathway by loss of function mutations in NF1, a gene which encodes the Ras-GTPase activating protein (GAP), neurofibromin (36). More recent evidence (9, 11, 37) has shown that several genes are amplified in a subset of MPNST cell lines along the 4q12 amplicon, some of which include genes for receptor tyrosine kinases such as PDGFR, VEGFR and c-Kit. Attempts have been made to identify “druggable” tyrosine kinases in MPNSTs (7, 38) and treatments have been targeted at down-regulating pathways that include Src, PDGFR, IGF-1R, c-Kit as well as c-Met and EGFR (9, 10, 20, 21, 39, 40).
PLX3397 is a novel multi-targeted tyrosine kinase inhibitor that is selective for the M-CSF receptor kinase (c-Fms) and c-Kit. PLX3397 has recently been reported to have activity against breast cancer (16), acute myeloid leukemia (41) as well as neurofibromas (17). Although, it is a known c-Fms inhibitor, the exact mechanism of action of this drug, especially in MPNST, has not been reported. In this study, we tested the effects of the drug on two MPNST cell lines that were derived from patients with MPNST tumors of the thigh, one of which was NF1-associated (18). Our results clearly demonstrate that PLX3397 inhibits in vitro cellular proliferation in MPNST cells. When we tested the activity of the drug in vitro using western blot analysis, we observed down regulation of c-Kit but not c-Fms even though both the MPNST cell lines express detectable levels of total c-Fms.
It has been recently demonstrated that tumor cell lines derived from NF1 patients, NF1 deficient cell lines, as well as spontaneous tumors arising in a genetically engineered mouse model with NF1 and TP53 mutations were highly sensitive to the mTOR inhibitor rapamycin (42, 43), arguing NF1 loss mediates oncogenesis through mTOR activation. To explore the effect of combined inhibition of RTK pathways and TORC1 signaling, we carried out an in vitro proliferation assay as well as analyzed cell lysates by western blots. Our results demonstrated a stronger inhibition of cellular proliferation when PLX3397 was combined with rapamycin. PLX3397 also induced G1-cell cycle arrest with down-regulation of cyclin D1 and p-Rb suggesting cytostatic rather than cytotoxic properties. Western blot analysis confirmed p-Kit and p-PDGFRβ down regulation irrespective of rapamycin combination. PDGFRβ has not been reported previously as a target for PLX3397. Rapamycin was able to down-regulate TORC1 effectors such as S6 but activated p-AKT through release of negative feedback inhibition (28). Combined blockade of RTKs and TORC1 pathway resulted in significantly better inhibition of MPNST cell proliferation compared to single agent treatments. This may be in part due to the fact that both imatinib and PLX3397 block the re-activation of p-AKT induced by rapamycin presumably mediated by PDGFRβ and c-Kit signaling.
Imatinib has been shown to inhibit c-Fms phosphorylation at various therapeutic concentrations (23, 24). Based on our in vitro data, the target specificity of imatinib and PLX3397 is highly overlapping. To test this specificity in vivo, we carried out MPNST mouse xenograft experiments using single agent or combination treatments. Interestingly, imatinib as a single agent or in combination with rapamycin was unable to maintain sustained inhibition of the target tyrosine kinases as well as AKT phosphorylation compared to PLX3397. PLX3397 was clearly superior in terms of sustained target inhibition compared to imatinib and also resulted in significant tumor volume suppression when the animals were treated with the drug alone or in combination with rapamycin.
c-Fms (M-CSF1R) kinase is involved in macrophage/microglial activation (33). Iba-1 (Ionized calcium binding adapter molecule 1), is a macrophage/microglia specific calcium-binding protein that has been shown to be involved in c-Fms mediated tumor growth (32). Macrophages are typically divided into two types: classically activated or M1 and alternatively activated or M2 (44). M1 macrophages are considered anti-tumorigenic, whereas, M2 macrophages are pro-tumorigenic (45) and are required for tumor vascularization, invasion and development. Novel therapeutic strategies aimed at targeting M2 or Tumor Associated Macrophages (TAMs) are currently being pursued with much interest (46). In a recent report (17), mouse models of neurofibromas showed that PLX3397 was highly effective in inhibiting macrophages in established tumors and therapeutically targeted macrophage depletion may result in impaired tumor maintenance.
Our immunohistochemical studies clearly demonstrated that PLX3397 was superior to imatinib in terms of macrophage depletion. Western blot analysis of xenograft lysates showed significant blockade of p-Fms as well as total c-Fms that was not seen in our in vitro studies strongly suggesting stromal down regulation of M-CSFR pathway in these tumors. Reduction in macrophage population can therefore be attributed to inhibition of c-Fms signaling in xenografts. Macrophage depletion could also have contributed to the reduction in total c-Fms levels detected on western blot. Surprisingly, mouse xenografts treated with a combination of PLX3397 and rapamycin showed decreased macrophage re-infiltration when the drug treatments were discontinued. A recent study reported that tumor associated macrophages (M2) are dependent on mTOR signaling and inhibition of mTOR signaling by rapamycin can block tumor growth and angiogenesis by preventing monocyte differentiation into M2 macrophages (47). Results from our Iba-1 staining and flow cytometric analysis indicated that rapamycin alone not only caused a decrease in macrophage population but also caused TAMs to shift from M2-like to more M1-like population partly explaining its potent anti-tumor effect in MPNST xenografts. Also, such a shift to M1-like population can possibly explain the observed lag time in tumor re-growth after PLX3397 and rapamycin treatment is discontinued compared to PLX3397 treatment alone. Sustained rapamycin treatment results in a switch to M1-like phenotype. This may cause a delay before macrophages become more M2-like again and support tumor growth when the drug treatments are discontinued. Studies targeting c-Fms inhibition resulted in macrophage depletion but such depletion alone was not sufficient to suppress tumor growth strongly suggesting a greater role played by RTKs in causing MPNST tumor growth and development.
Taken together, our data strongly suggests that sustained inhibition of RTK signaling pathways by PLX3397 in vivo makes it superior to imatinib, even though the target specificity of the two drugs is overlapping. Furthermore, even though neither imatinib nor PLX3397 inhibited p-Fms in vitro, only PLX3397 could inhibit p-Fms in vivo. This resulted in a marked depletion of macrophages and contributed to its significant anti-tumor effect in vivo. In addition, rapamycin was able to sustain this effect by decreasing cell proliferation and promoting further macrophage depletion as part of the combination therapy. PLX3397 is currently in clinical trials for various types of cancers including lymphoma, glioblastoma and metastatic breast cancer. Our results indicate that PLX3397 in combination with an mTOR inhibitor should be considered the next step in drug development for patients with MPNST.
Supplementary Material
Translational Relevance.
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive cancers that have gene amplification of specific receptor tyrosine kinases (RTKs) including PDGFR as well as c-Kit. Here we report the effect of the c-Kit inhibitor imatinib and the selective c-Fms and c-Kit inhibitor PLX3397, both alone and in combination with the TORC1 inhibitor rapamycin, against MPNST cells. Our data strongly suggests that sustained inhibition of RTK signaling pathways by PLX3397 in vivo makes it superior to imatinib, even though the target specificity of the two drugs is comparable. Only PLX3397 could inhibit p-Fms in vivo resulting in a marked depletion of macrophages and significant tumor suppression in vivo. Furthermore, rapamycin was able to sustain tumor suppression by decreasing cell proliferation and promoting further macrophage depletion as part of the combination therapy. Our study can be translated into the development of PLX3397 and TORC1 inhibitor combination therapy for patients with MPNST.
Acknowledgments
Financial Support: P50 CA140146 (Gary K. Schwartz)
CA102613, T32 CA09501 (Ronald P. DeMatteo)
T32 CA09501 (Michael J. Beckman)
Footnotes
The authors have no conflicts to disclose in relation to this work.
References
- 1.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–7. [PubMed] [Google Scholar]
- 2.Ferner RE. Neurofibromatosis 1. Eur J Hum Genet. 2007;15:131–8. doi: 10.1038/sj.ejhg.5201676. [DOI] [PubMed] [Google Scholar]
- 3.Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13:2–7. doi: 10.1016/j.spen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Evans DG, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–4. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCaughan JA, Holloway SM, Davidson R, Lam WW. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44:463–6. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin Liberal J, Lagares-Tena L, Sainz-Jaspeado M, Mateo-Lozano S, Garcia Del Muro X, Tirado OM. Targeted therapies in sarcomas: challenging the challenge. Sarcoma. 2012:626094. doi: 10.1155/2012/626094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shor AC, Agresta SV, D’Amato GZ, Sondak VK. Therapeutic potential of directed tyrosine kinase inhibitor therapy in sarcomas. Cancer Control. 2008;15:47–54. doi: 10.1177/107327480801500106. [DOI] [PubMed] [Google Scholar]
- 8.Demestre M, Herzberg J, Holtkamp N, Hagel C, Reuss D, Friedrich RE, et al. Imatinib mesylate (Glivec) inhibits Schwann cell viability and reduces the size of human plexiform neurofibroma in a xenograft model. J Neurooncol. 98:11–9. doi: 10.1007/s11060-009-0049-4. [DOI] [PubMed] [Google Scholar]
- 9.Holtkamp N, Okuducu AF, Mucha J, Afanasieva A, Hartmann C, Atallah I, et al. Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis. 2006;27:664–71. doi: 10.1093/carcin/bgi273. [DOI] [PubMed] [Google Scholar]
- 10.Aoki M, Nabeshima K, Koga K, Hamasaki M, Suzumiya J, Tamura K, et al. Imatinib mesylate inhibits cell invasion of malignant peripheral nerve sheath tumor induced by platelet-derived growth factor-BB. Lab Invest. 2007;87:767–79. doi: 10.1038/labinvest.3700591. [DOI] [PubMed] [Google Scholar]
- 11.Zietsch J, Ziegenhagen N, Heppner FL, Reuss D, von Deimling A, Holtkamp N. The 4q12 amplicon in malignant peripheral nerve sheath tumors: consequences on gene expression and implications for sunitinib treatment. PLoS One. 5:e11858. doi: 10.1371/journal.pone.0011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 11:865–78. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 13.Lasota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diagn Pathol. 2006;23:91–102. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Robertson KA, Nalepa G, Yang FC, Bowers DC, Ho CY, Hutchins GD, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: a phase 2 trial. Lancet Oncol. 13:1218–24. doi: 10.1016/S1470-2045(12)70414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14:4550–5. doi: 10.1158/1078-0432.CCR-08-0086. [DOI] [PubMed] [Google Scholar]
- 16.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prada CE, Jousma E, Rizvi TA, Wu J, Dunn RS, Mayes DA, et al. Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathol. 125:159–68. doi: 10.1007/s00401-012-1056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambrosini G, Cheema HS, Seelman S, Teed A, Sambol EB, Singer S, et al. Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther. 2008;7:890–6. doi: 10.1158/1535-7163.MCT-07-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambol EB, Ambrosini G, Geha RC, Kennealey PT, Decarolis P, O’Connor R, et al. Flavopiridol targets c-KIT transcription and induces apoptosis in gastrointestinal stromal tumor cells. Cancer Res. 2006;66:5858–66. doi: 10.1158/0008-5472.CAN-05-2933. [DOI] [PubMed] [Google Scholar]
- 20.Torres KE, Zhu QS, Bill K, Lopez G, Ghadimi MP, Xie X, et al. Activated MET is a molecular prognosticator and potential therapeutic target for malignant peripheral nerve sheath tumors. Clin Cancer Res. 17:3943–55. doi: 10.1158/1078-0432.CCR-11-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Ylipaa A, Sun Y, Zheng H, Chen K, Nykter M, et al. Genomic and molecular characterization of malignant peripheral nerve sheath tumor identifies the IGF1R pathway as a primary target for treatment. Clin Cancer Res. 17:7563–73. doi: 10.1158/1078-0432.CCR-11-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, et al. KIT oncogene inhibition drives intratumoral macrophage M2 polarization. The Journal of experimental medicine. 2013;210:2873–86. doi: 10.1084/jem.20130875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewar AL, Zannettino AC, Hughes TP, Lyons AB. Inhibition of c-fms by imatinib: expanding the spectrum of treatment. Cell Cycle. 2005;4:851–3. doi: 10.4161/cc.4.7.1788. [DOI] [PubMed] [Google Scholar]
- 24.Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–32. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 26.Blay JY. Updating progress in sarcoma therapy with mTOR inhibitors. Ann Oncol. 22:280–7. doi: 10.1093/annonc/mdq307. [DOI] [PubMed] [Google Scholar]
- 27.Agulnik M. New developments in mammalian target of rapamycin inhibitors for the treatment of sarcoma. Cancer. 118:1486–97. doi: 10.1002/cncr.26361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rixe O, Fojo T. Is cell death a critical end point for anticancer therapies or is cytostasis sufficient? Clin Cancer Res. 2007;13:7280–7. doi: 10.1158/1078-0432.CCR-07-2141. [DOI] [PubMed] [Google Scholar]
- 30.Masamha CP, Benbrook DM. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009;69:6565–72. doi: 10.1158/0008-5472.CAN-09-0913. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Zhou J, Fan J, Qiu SJ, Yu Y, Huang XW, et al. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14:5124–30. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 32.Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–74. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- 33.Sweet MJ, Hume DA. CSF-1 as a regulator of macrophage activation and immune responses. Arch Immunol Ther Exp (Warsz) 2003;51:169–77. [PubMed] [Google Scholar]
- 34.Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol Med. 18:519–27. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercalli A, Calavita I, Dugnani E, Citro A, Cantarelli E, Nano R, et al. Rapamycin unbalances the polarization of human macrophages to M1. Immunology. 2013;140:179–90. doi: 10.1111/imm.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kraniak JM, Sun D, Mattingly RR, Reiners JJ, Jr, Tainsky MA. The role of neurofibromin in N-Ras mediated AP-1 regulation in malignant peripheral nerve sheath tumors. Mol Cell Biochem. 344:267–76. doi: 10.1007/s11010-010-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrone F, Da Riva L, Orsenigo M, Losa M, Jocolle G, Millefanti C, et al. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol. 2009;11:725–36. doi: 10.1215/15228517-2009-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres KE, Liu J, Young E, Huang KL, Ghadimi M, Lusby K, et al. Expression of ‘drugable’ tyrosine kinase receptors in malignant peripheral nerve sheath tumour: potential molecular therapeutic targets for a chemoresistant cancer. Histopathology. 59:156–9. doi: 10.1111/j.1365-2559.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shor AC, Keschman EA, Lee FY, Muro-Cacho C, Letson GD, Trent JC, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67:2800–8. doi: 10.1158/0008-5472.CAN-06-3469. [DOI] [PubMed] [Google Scholar]
- 40.Holtkamp N, Malzer E, Zietsch J, Okuducu AF, Mucha J, Mawrin C, et al. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro Oncol. 2008;10:946–57. doi: 10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hatzimichael E, Georgiou G, Benetatos L, Briasoulis E. Gene mutations and molecularly targeted therapies in acute myeloid leukemia. Am J Blood Res. 3:29–51. [PMC free article] [PubMed] [Google Scholar]
- 42.Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, et al. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 43.Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohno S, Suzuki N, Ohno Y, Inagawa H, Soma G, Inoue M. Tumor-associated macrophages: foe or accomplice of tumors? Anticancer Res. 2003;23:4395–409. [PubMed] [Google Scholar]
- 46.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, et al. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 72:1363–72. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.