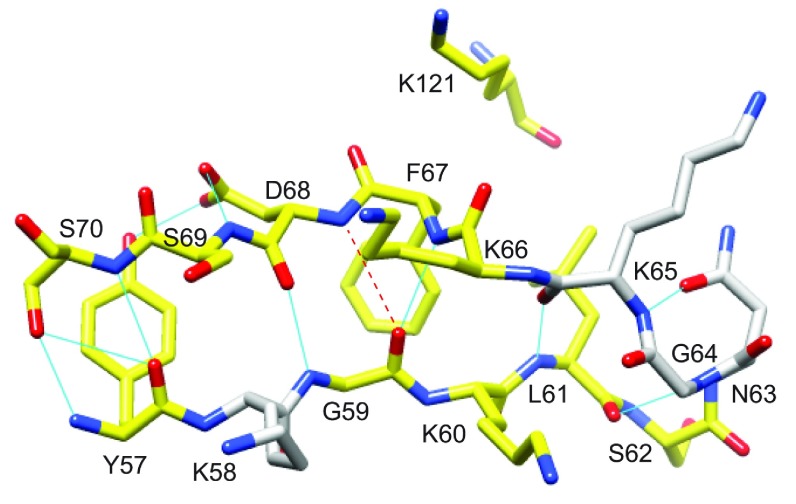
Figure 7. Structural distribution of residues in the β2 and β3a strands of FKBP51 that exhibit reductions in R2 values resulting from the L119P substitution.
Residues for which the 15N R2 value decreases by more than 0.5 s−1 at 900 MHz 1H are coloured yellow. There are no other differences in R2 greater than 0.5 s−1 outside the β4–β5 loop. A kink in the β3a strand occurs at Phe67 and Asp68 where the amide hydrogen of Asp68 is slightly too far from the carbonyl oxygen of Gly59 to form a canonical antiparallel β-sheet hydrogen-bonding interaction. This kink occurs at the site of direct contact with the tip of the β4–β5 loop as indicated by Lys121.