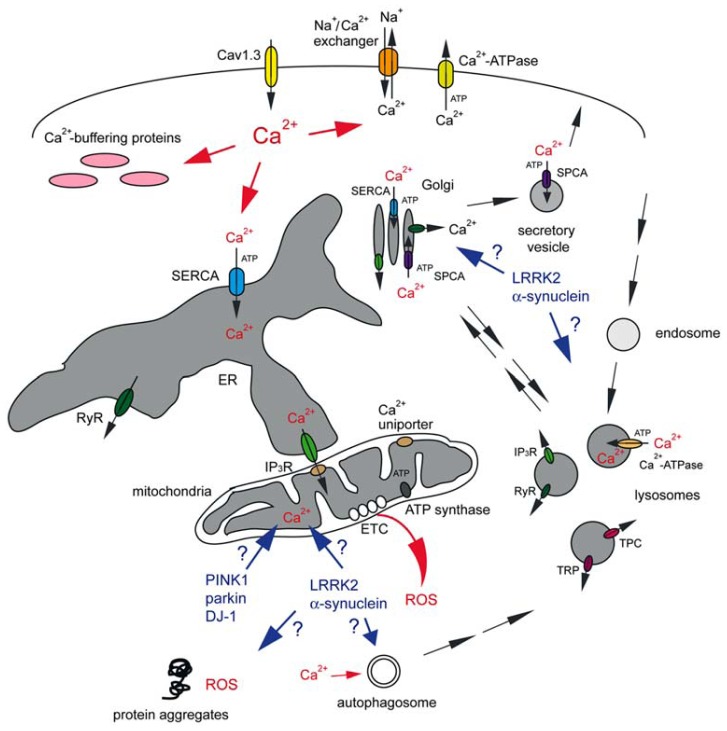
FIGURE 1.
Abnormal Ca2+ signaling in SNc DA neurons may cause mitochondrial oxidant stress, proteostasis deficits and eventual cell death. Vulnerable neuronal populations display spontaneous slow pacemaking activity employing Cav1.3 L-type Ca2+ channels, prominent Ca2+ currents and low intrinsic Ca2+ buffering capacities. Ca2+ inside the neuron can be transported back across the plasma membrane either via plasma membrane Ca2+-ATPase at the cost of ATP consumption, or through the Na+/Ca2+ exchanger which uses the Na+ gradient across the plasma membrane. Ca2+ is rapidly sequestered by interactions with Ca2+ buffering proteins or taken up into a variety of intracellular organelles. The ER uses a high-affinity Ca2+-ATPase [the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA)] to pump Ca2+ into the ER lumen at the cost of ATP consumption. This pump is also present on cis and medial Golgi membranes, whilst secretory vesicles employ a secretory pathway Ca2+-ATPase (SPCA) which is also be present on the trans Golgi complex. Ca2+ uptake into acidic organelles is mediated by a molecularly unidentified Ca2+-ATPase. Ca2+ flows back into the cytosol from the ER lumen through IP3 receptors (IP3R) or ryanodine receptors (RyR). IP3R are also present on cis and medial Golgi membranes, RyR on trans Golgi membranes, and RyR, TRP and TPC channels are present on acidic organelles. Mitochondria, often in close apposition to the ER or plasma membrane, can take up Ca2+ into the matrix through a mitochondrial Ca2+ uniporter. Ca2+ transfer between ER and mitochondria involves the IP3R on the ER membrane. Ca2+ within mitochondria is necessary for proper ETC function to generate ATP by ATP synthase, but mitochondrial Ca2+ overload can cause mitochondrial oxidant stress (ROS). Toxins as well as familial mutations in PINK1, parkin and DJ-1 affect mitochondrial ATP production and Ca2+ handling, even though the molecular details remain to be determined. The effects of familial mutations in LRRK2 and α-synuclein on mitochondrial functioning are even less clear, but those mutant proteins may cause additional deficits in proteostasis through mechanisms involving Ca2+-regulated events such as autophagy. This may also include alterations in the trafficking of Golgi-derived vesicles to the plasma membrane, resulting in changes in vesicle secretion and in the steady-state levels of surface receptors. Golgi deficits may cause altered trafficking of enzymes destined to lysosomes, with concomitant deficits in lysosomal degradative capacity, or alterations in retromer-mediated retrieval from endolysosomes back to the Golgi. Finally, changes in acidic store Ca2+ levels may affect various endo-lysosomal trafficking steps or the degradative capacity of acidic organelles per se. For further details see text.