Figure 9. Structure and aggregating behavior of TP10.
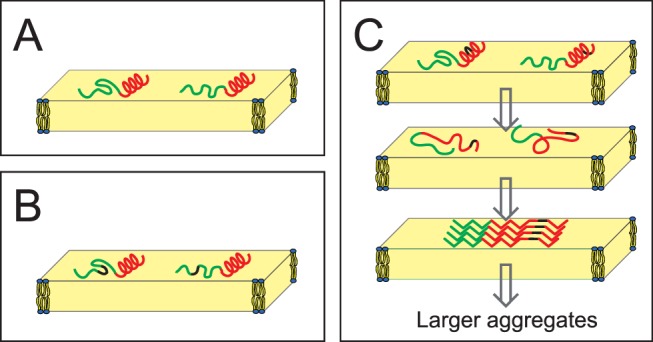
(A) Monomeric structure of TP10 as determined from 19F-NMR in the membrane-bound state. The N-terminal galanin-derived region (green) is intrinsically unstructured, while the mastoparan-derived C-terminus (red) is folded as an α-helix. (B) When D -CF3-Bpg (black) is introduced into the flexible region, there is no conformational change. (C) However, when this stiff D -amino acid is placed into the helical C-terminal region, it leads to unfolding. At low concentration the monomers remain unstructured, but at high concentration surface-induced aggregation leads to the assembly of β-pleated amyloid-like fibrils. These cross-β-sheet assemblies must be distorted in the immediate vicinity of the D -amino acid. There is no indication if the β-sheets are parallel or antiparallel. As the β-sheets have no preferential orientation in the lipid bilayer (and they may be twisted), they give powder-type NMR spectra.
