Abstract
Patient: Female, 14 months
Final Diagnosis: Slit ventricle syndrome
Symptoms: Hydrocephalus • lethargy and seizure • vomiting
Medication: —
Clinical Procedure: —
Specialty: Pediatrics and Neonatology
Objective:
Challenging differential diagnosis
Background:
Shunt surgery is a common solution for hydrocephalus in infancy. Slit ventricle syndrome and secondary craniosynostosis are late-onset complications after shunt placement; these 2 conditions occasionally occur together.
Case Report:
We report a case of early-onset secondary craniosynostosis with slit ventricle syndrome after shunt surgery in an infant, which led to a catastrophic increase in intracranial pressure (ICP). A 4-month-old girl with a Dandy-Walker malformation underwent a ventriculoperitoneal shunt procedure. Her head circumference (HC) gradually decreased to approximately the 5th percentile for her age group after shunt surgery. Seven months later, she developed increased ICP symptoms and underwent a shunt revision with a diagnosis of shunt malfunction. Her symptoms were temporarily relieved, but she repeatedly visited the emergency room (ER) for the same symptoms and finally collapsed, with an abrupt increase in ICP, 3 months later. Further evaluation revealed the emergence of sagittal synostosis at 7 months after initial shunt surgery. After reviewing all clinical data, slit ventricle syndrome combined with secondary craniosynostosis was diagnosed. Emergent cranial expansion surgery with shunt revision was performed, and the increased ICP signs subsided in the following days.
Conclusions:
Clinical suspicion and long-term HC monitoring are important in the diagnosis of slit ventricle syndrome and secondary craniosynostosis after shunt surgery, even in infants and young children.
MeSH Keywords: Cerebrospinal Fluid Shunts, Craniosynostosis, Hydrocephalus, Slit Ventricle Syndrome
Background
Shunt surgery is a common treatment for hydrocephalus in infancy. Shunt surgery provides immediate relief of intracranial pressure (ICP) and its symptoms in affected children. However, shunting distorts normal cerebrospinal fluid (CSF) dynamics to such an extent that many problems may develop after the procedure [1]. Overdrainage of CSF can affect the physical characteristics and growth of the brain parenchyma, ventricles, and skull [2]. A decrease in ventricular size and brain compliance occasionally leads to slit ventricle syndrome, a serious complication of long-standing shunts [3,4]. In slit ventricle syndrome, small ventricles and a stiff brain make the shunt malfunction and cause significant headache and other symptoms of increased ICP. Derangement of skull growth results in secondary craniosynostosis and small head circumference (HC). It is known that secondary craniosynostosis develops in 1–5% of patients with shunts [5,6]. Most patients with secondary craniosynostosis are asymptomatic, but the condition is occasionally combined with slit ventricle syndrome [2]. In this situation, the 2 conditions can elicit a significant and synergistic increase in ICP and its symptoms, requiring urgent surgical intervention [2].
However, these changes in the brain and skull usually develop from long-standing shunts, usually years after shunt surgery [7]. Early-onset slit ventricle syndrome and secondary craniosynostosis are extremely rare in infants who underwent shunt surgery only months earlier. The symptoms and signs of slit ventricle syndrome and secondary craniosynostosis overlap with those of shunt obstruction, which is far more common. Therefore, differential diagnosis of these conditions in infants and young children may require clinical acumen.
We encountered an infant who developed recurring shunt malfunctions from age 11 months, only 7 months after initial shunt surgery. She eventually had a catastrophic increase in ICP, and emergent cranial vault expansion surgery and shunt revision saved her life. This report demonstrates the importance of clinical suspicion and long-term HC monitoring in the diagnosis of rare complications of shunt surgery in an unusual time period.
Case Report
A 4-month-old girl visited the emergency room (ER) due to irritability and projectile vomiting. Her HC was 41.5 cm (50th–75th percentile), and bulging of the anterior fontanel was observed. Brain computerized tomography (CT) and magnetic resonance imaging (MRI) showed a Dandy-Walker malformation with hydrocephalus (Figure 1A and 1B). A ventriculoperitoneal shunt was installed in the right lateral ventricle. One month after shunt surgery (age 5 months), the patient was stable, and a decompressed ventricle was observed on brain ultrasonography. Her HC was 41 cm (25th–50th percentile). Five months after shunt surgery (age 9 months), the patient developed well, without specific problems, but her HC was 41.6 cm, stagnating at the 3th–5th percentile for her age group. A follow-up CT scan showed small lateral ventricles (Figure 1C) and decreased size for the 4th ventricular cyst.
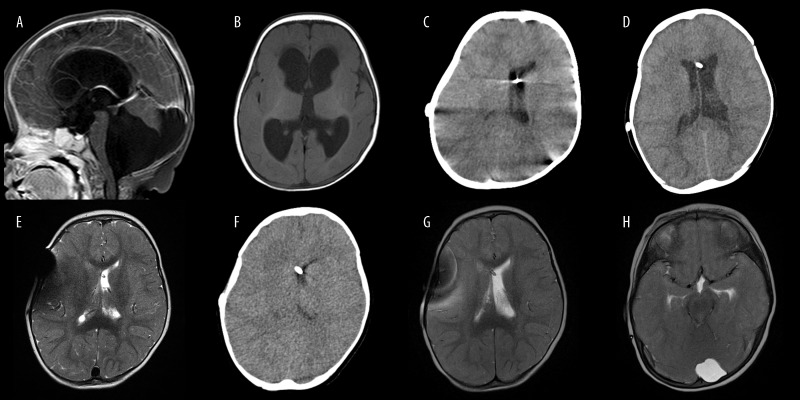
Figure 1.
(A, B) Initial T1-weighted MR images of the patient’s brain, including a Dandy-Walker malformation with hydrocephalus. (C) Brain CT images at 5 months after shunt surgery, showing decreased size of lateral ventricles. (D) Brain CT images at 7 months after shunt surgery, presenting slightly dilated lateral ventricles. (E) T2-weighted MR image at 9 months after shunt surgery, with an obliterated right lateral ventricle and a small left lateral ventricle. (F) Brain CT image and (G) T2-weighted MR images at 10 months after shunt surgery, with catastrophic manifestation. The left lateral ventricle is slightly expanded, but the right lateral ventricle appears to be slit-like. (H) A sign of impending bilateral uncal herniation appears in T2-weighted MR images.
Seven months after shunt surgery (age 11 months), the patient visited the ER due to vomiting that lasted for 2 days. Her HC measured 42.5 cm (5th–10th percentile), and bulging of the anterior fontanel was noted. A CT scan showed slight dilatation of both lateral ventricles and the 4th ventricle cyst (Figure 1D). Shunt reservoir tapping yielded no aspiration of CSF. Proximal shunt obstruction was diagnosed, and the shunt was revised immediately. The patient’s symptoms disappeared after shunt revision, and she was discharged.
Two months later (age 13 months), the patient visited the ER again for sudden recurrent vomiting. The anterior fontanel was narrow on palpation, but filling of the shunt reservoir was intact. MRI revealed a slit-like right lateral ventricle and a small left lateral ventricle (Figure 1E). The patient stopped vomiting after symptomatic management and was discharged without further evaluation.
One month later (age 14 months), the patient revisited the ER for vomiting and poor oral intake. She was lethargic at presentation. Cranial nerve exams were initially normal, and there was no lateralizing sign. Her limb muscle tone was decreased but symmetric. Her HC was 43.0 cm (below the 3rd percentile), and the anterior fontanel was nearly closed. Shunt reservoir filling was poor, and no CSF was drained from the shunt tap. The initial serum sodium and potassium levels were 136 mEq/L (normal range: 135–145 mEq/L) and 4.1 mEq/L (normal range: 3.5–5.0 mEq/L), respectively. Brain CT and MRI showed a slightly expanded left lateral ventricle. The right lateral ventricle was still small (Figure 1F and 1G). Nearly every sulcus of the cerebral hemispheres was obliterated, and perimesencephalic cisterns were invisible, which indicated impending bilateral uncal herniation (Figure 1H). During the work-up, the patient developed generalized tonic-clonic seizures. Her serum sodium level was 116 mEq/L, and her potassium level was 3.7 mEq/L, as determined after the seizure attacks. Both the hyponatremia and the seizures were considered as acute manifestations of profound encephalopathy and increased ICP.
A proximal shunt obstruction was again suspected because of the slightly expanded left lateral ventricle and the dry shunt tap. However, the right lateral ventricle was completely obliterated where the majority of proximal catheter holes were located. The slit ventricle mechanism might have been working, disabling the shunt system. Reviewing the radiological images, we found that the patient’s sagittal suture was invisible on the CT scan. 3D-CT images confirmed the closure of a sagittal suture. We re-evaluated all previous CT scans, and the closure of the sagittal suture began at 7 months after initial shunt surgery (at the time of the previous shunt revision) (Figure 2). Slit ventricle syndrome combined with secondary craniosynostosis was diagnosed. It was not clear whether the shunt malfunction was temporary or permanent. Emergent cranial expansion surgery and shunt revision were performed to relieve the ICP and to restore shunt function. The closed sagittal suture was opened wide, and several barrel-stave osteotomies were made coronally. The shunt proximal catheter and valve were replaced simultaneously (Figure 3).
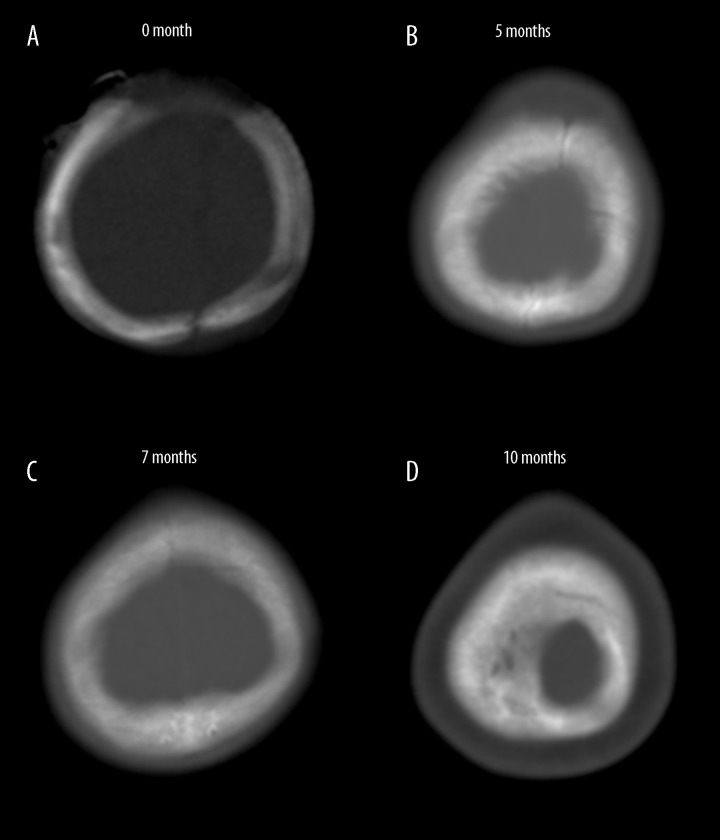
Figure 2.
Retrospective review of the brain CT scans at (A) 0 months, (B) 5 months, (C) 7 months, and (D) 10 months after the initial shunt operation. The sagittal suture began to close at 7 months after initial shunt surgery (at the time of the first shunt revision).
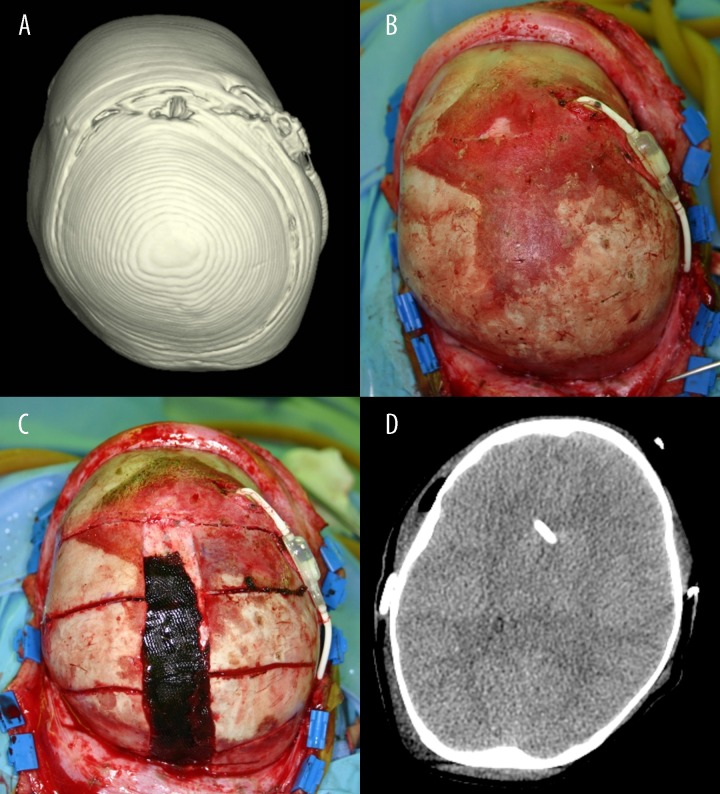
Figure 3.
(A) 3D-CT brain image and (B) intraoperative photograph taken before cranial expansion surgery, with the same orientation. The closed sagittal suture is clearly visible on both images. (C) Sagittal suturectomy with barrel-stave osteotomy was performed. (D) Postoperative brain CT image 1 day after cranial expansion surgery. The ventricles and sulci are all effaced.
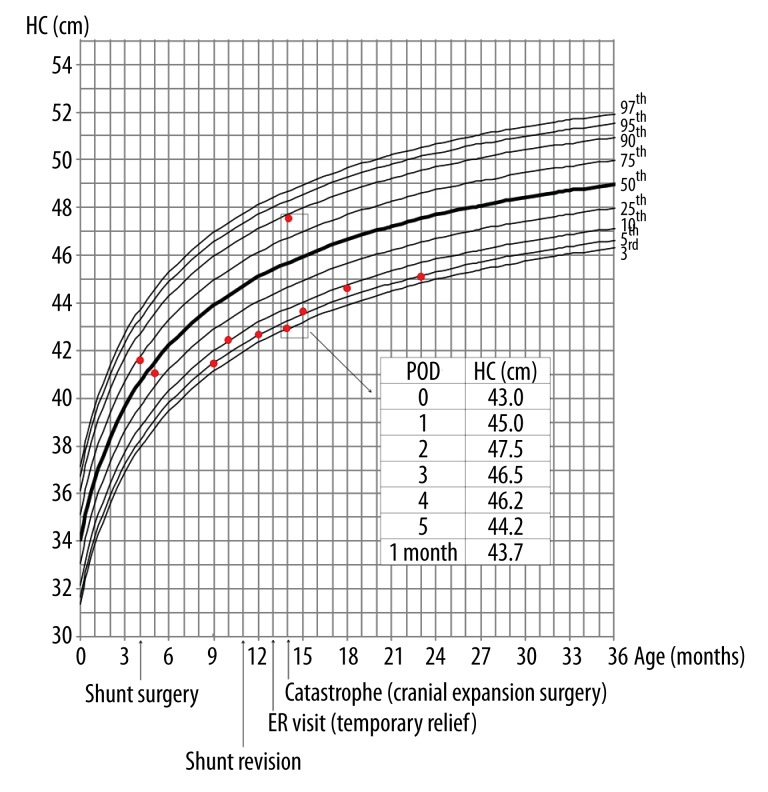
Immediately after the operation, the patient’s HC increased from 43.0 cm to 45.0 cm. At postoperative day (POD) 2, her HC increased to 47.5 cm. She was still lethargic, with little spontaneous activity. A CT scan showed effacement of all cerebral sulci and ventricles. Mannitol was infused every 4 hours to reduce brain swelling. At POD 3, the patient’s HC decreased to 46.5 cm, and she became alert, with spontaneous activity. Her HC decreased gradually thereafter, and she was discharged at POD 8. One month later, she was healthy, without neurological sequelae, and her HC was 43.7 cm (5th–10th percentile), slightly greater than the preoperative level. Nine months after cranial expansion surgery (age 23 months), she could walk without assistance, climb up stairs, and play with puzzles, and her HC was 45.2 cm (3th–5th percentile) (Figure 4).
Figure 4.
The transition of the patient’s HC on a standard Korean girl’s head circumference chart (marked with red dots) [15]. Dramatic changes in HC around the cranial expansion surgery are described in the table. The patient’s HC declined after shunt surgery and remained small thereafter.
Discussion
Shunt obstruction (failure) is the usual presumptive diagnosis when a child with a shunt develops symptoms of increased ICP. Imaging studies and shunt tapping may be the next step for the diagnosis of shunt obstruction. However, the background etiology of recurrent shunt failures is frequently unnoticed in clinical practice; slit ventricle syndrome is an example of this. Symptoms of shunt obstruction, slit-like ventricles in imaging studies, and slow filling of the shunt reservoir are the classical triad of slit ventricle syndrome [4]. Nonetheless, the intermittent nature of symptoms, subtle changes in ventricle size, and inexperience of physicians often lead to the misdiagnosis of simple shunt obstruction. Weinzweig et al. [2] reported that an average of 4.9 shunt revisions are performed in children with slit ventricle syndrome and secondary craniosynostosis until they receive cranial vault expansion.
Secondary craniosynostosis is the premature fusion of cranial sutures and is often found after shunt surgery. This phenomenon is uncommon and usually asymptomatic. Therefore, clinicians frequently pay little attention to the cranial vault of shunted children, omitting to confirm suture lines when reviewing routine follow-up imaging. This pitfall makes inexperienced clinicians fail to recognize secondary craniosynostosis in shunted children. Although uncommon and usually asymptomatic, this condition can be combined with slit ventricle syndrome and cause a significant increase in ICP. Shunt revision may alleviate the symptoms temporarily, but cranial vault expansion is required to address the complex situation [2,8].
In the present case, certain notable features should be mentioned. First, craniosynostosis (with a slit ventricle mechanism) began only 7 months and became overtly symptomatic 9 months after initial shunt placement. Such an early onset of suture fusion by shunt surgery is unexpected, and this possibility was unheeded. Pudenz and Foltz suggested an interval of 2–3 years for the development of premature suture fusion after initial shunt surgery [7]. Weinzweig et al. [2] reported an average interval of 26 months for the development of craniosynostosis. However, several authors reported that secondary craniosynostosis was detected in infants several months after shunt surgery, indicating that suture fusion after shunt surgery may begin earlier in certain patients [9,10]. Slit ventricle syndrome is also a complication of a long-standing shunt, as the pathophysiology may include complex changes in the physical properties of the brain and skull [11,12]. In a review by Pudenz and Folz [7], an interval of 4–10 years was indicated.
Second, the presentation of this condition was catastrophic, with an abrupt elevation of ICP and neurological collapse. The cardinal symptom of slit ventricle syndrome is chronic headache. It is possible that patients with slit ventricle syndrome and headache show very high ICP upon pressure monitoring. Nonetheless, the chronic nature of the condition may prevent acute collapse of the patients, even those with high ICP. In our patient, slit ventricle syndrome developed in infancy, when the brain is still growing rapidly. This unusually early manifestation and the combined secondary craniosynostosis may have contributed to the dramatic manifestation.
Third, it is instructive that the patient visited the ER twice before the catastrophe. During the first ER visit, she was diagnosed with shunt obstruction. The diagnosis of shunt obstruction was most likely correct. However, the ventricular dilatation was not sufficiently severe at that time to indicate proximal shunt obstruction. We also failed to recognize the underlying problems of the patient, focusing only on simple shunt obstruction. Reviewing all CT images retrospectively, we found that the sagittal suture closure had already begun at the time of initial shunt revision. The patient even visited the ER again after shunt revision with the same symptoms and experienced spontaneous symptom resolution, a typical course of slit ventricle syndrome. Therefore, cranial volume restriction combined with slit ventricle syndrome may be the real diagnosis causing recurrent ER visits.
Fourth, this case demonstrates the utmost importance of serial HC measurement in pediatric neurosurgical practices. The patient’s HC decreased far more than expected after shunt surgery and remained extremely small. Looking carefully at the HC curve may lead to questions about and appropriate evaluation of secondary craniosynostosis.
Lastly, the dramatic change in HC after cranial expansion surgery reflected the physical elastance of the brain. In the immediate postoperative days, the patient’s HC increased by approximately 2 cm per day and gradually stabilized after POD 3. The changes in her consciousness and neurological status correlated well with the variation of HC. This patient exhibited multiple pathological mechanisms, including cranial volume restriction, slit ventricle syndrome, and shunt malfunction. The cranial expansion procedure may be effective for treating slit ventricle syndrome and secondary craniosynostosis. For proper treatment, both cranial expansion and shunt revision are required to cover all of these pathological mechanisms.
Suturectomy evolved as the main treatment method for secondary craniosynostosis since the 1980s [13]. Recently, several modern techniques, such as spring-assisted remodeling and distraction devices, have been applied in secondary craniosynostosis to achieve better outcomes [9,14]. However, suturectomy is a very useful and versatile method in emergent situations in which a rapid reduction in ICP is warranted. In our patient, suturectomy and barrel-stave osteotomy induced an immediate increase in HC and the relief of neurological symptoms. Therefore, surgical methods for cranial expansion should be determined according to the patient’s clinical manifestation.
To prevent this complication, CSF overdrainage should be avoided as much as possible. The setting of a programmable shunt should be set to high to prevent slit-like ventricles and premature suture fusion. The importance of HC monitoring needs to be emphasized. Manual examination and a skull X-ray are required to assess the status of suture lines during follow-ups.
Conclusions
The early appearance of secondary craniosynostosis with a slit ventricle mechanism and catastrophic manifestations was remarkable, and the physical elastance of the brain was well documented in the clinical course of this patient. This report demonstrates the importance of clinical suspicion and long-term HC monitoring in the diagnosis of slit ventricle syndrome and secondary craniosynostosis after shunt surgery, even in infants and young children.
Footnotes
Source of support: This study was supported by a grant from Seoul National University Hospital (No. 0420130930; to Phi JH)
References:
- 1.Di Rocco C, Marchese E, Velardi F. A survey of the first complication of newly implanted CSF shunt devices for the treatment of nontumoral hydrocephalus. Cooperative survey of the 1991–1992 Education Committee of the ISPN. Childs Nerv Syst. 1994;10:321–27. doi: 10.1007/BF00335171. [DOI] [PubMed] [Google Scholar]
- 2.Weinzweig J, Bartlett SP, Chen JC, et al. Cranial vault expansion in the management of postshunt craniosynostosis and slit ventricle syndrome. Plast Reconstr Surg. 2008;122:1171–80. doi: 10.1097/PRS.0b013e3181858c84. [DOI] [PubMed] [Google Scholar]
- 3.Kiekens R, Mortier W, Pothmann R, et al. The slit-ventricle syndrome after shunting in hydrocephalic children. Neuropediatrics. 1982;13:190–94. doi: 10.1055/s-2008-1059621. [DOI] [PubMed] [Google Scholar]
- 4.Olson S. The problematic slit ventricle syndrome. A review of the literature and proposed algorithm for treatment. Pediatr Neurosurg. 2004;40:264–69. doi: 10.1159/000083738. [DOI] [PubMed] [Google Scholar]
- 5.Faulhauer K, Schmitz P. Overdrainage phenomena in shunt treated hydrocephalus. Acta Neurochir (Wien) 1978;45:89–101. doi: 10.1007/BF01774384. [DOI] [PubMed] [Google Scholar]
- 6.Roberts JR, Rickham PP. Craniostenosis following Holter valve operation. Dev Med Child Neurol. 1970;12(Suppl.22):145–49. doi: 10.1111/j.1469-8749.1970.tb03018.x. [DOI] [PubMed] [Google Scholar]
- 7.Pudenz RH, Foltz EL. Hydrocephalus: Overdrainage by ventricular shunts. A review and recommendations. Surgl Neurol. 1991;35:200–12. doi: 10.1016/0090-3019(91)90072-h. [DOI] [PubMed] [Google Scholar]
- 8.Baird LC, Gonda D, Cohen SR, et al. Craniofacial reconstruction as a treatment for elevated intracranial pressure. Childs Nerv Syst. 2012;28:411–18. doi: 10.1007/s00381-011-1615-6. [DOI] [PubMed] [Google Scholar]
- 9.Park DH, Chung J, Yoon SH. The role of distraction osteogenesis in children with secondary craniosynostosis after shunt operation in early infancy. Pediatr Neurosurg. 2009;45:437–45. doi: 10.1159/000277618. [DOI] [PubMed] [Google Scholar]
- 10.Shuster BA, Norbash AM, Schendel SA. Correction of scaphocephaly secondary to ventricular shunting procedures. Plast Reconstr Surg. 1995;96:1012–19. doi: 10.1097/00006534-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman B, Weiss MH, Young HF, Nulsen FE. Effects of prolonged cerebrospinal fluid shunting on the skull and brain. J Neurosurg. 1973;38:288–97. doi: 10.3171/jns.1973.38.3.0288. [DOI] [PubMed] [Google Scholar]
- 12.Rekate HL. The slit ventricle syndrome: advances based on technology and understanding. Pediatr Neurosurg. 2004;40:259–63. doi: 10.1159/000083737. [DOI] [PubMed] [Google Scholar]
- 13.Kloss JL. Craniosynostosis secondary to ventriculoatrial shunt. Am J Dis Child. 1968;116:315–17. doi: 10.1001/archpedi.1968.02100020317015. [DOI] [PubMed] [Google Scholar]
- 14.Davis C, Lauritzen CGK. Spring-assisted remodeling for ventricular shunt-induced cranial deformity. J Craniofac Surg. 2008;19:588–92. doi: 10.1097/SCS.0b013e31816aaa60. [DOI] [PubMed] [Google Scholar]
- 15.Korea Centers for Disease Control and Prevention and Korean Pediatric Society: 2007 Standard growth chart of children and adolescents [cited 24 Feb 2014] Available from: URL: http://www.cdc.go.kr/CDC/notice/CdcKrTogether0302.jsp?menuIds=HOME001-MNU1154-MNU0004-MNU0088&cid=9838.