Figure 2.
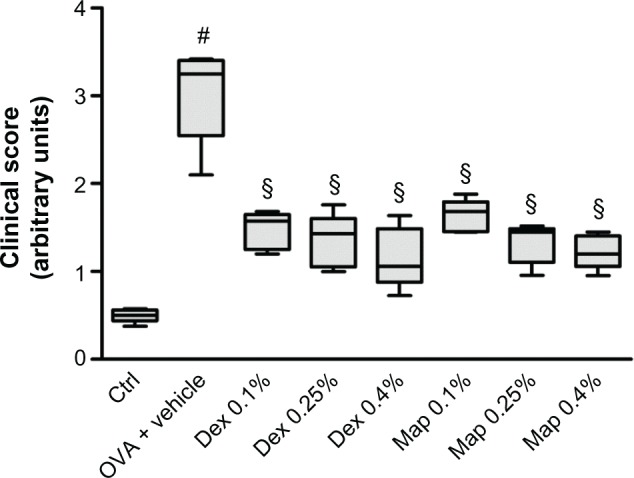
Map and Dex inhibit the clinical signs of late-phase conjunctival allergic inflammation to OVA in sensitized guinea pigs.
Notes: Map and Dex eye drops (0.1%, 0.25%, and 0.4%; w/v) or the vehicle (30 μL/eye) were administered in the conjunctival sac of both eyes 2 hours after OVA challenge (30 μL of 2.5% solution). Ctrls received the vehicle alone and were not treated with topical OVA. Each group comprised five guinea pigs, and the score, evaluated on both eyes (n=10) was based on changes observed 4, 6, and 8 hours after OVA challenge (2, 4, and 6 hours after drug treatment) for the symptoms of itching, swelling, redness, and lid eversion and was rated in a masked fashion using the following scale: 0, no symptoms; 1, slight conjunctival redness with or without tears; 2, mild conjunctival redness with or without tears and mild chemosis; 3, mild conjunctival redness with or without tears and moderate chemosis; 4, severe conjunctival redness with tears and partial lid eversion; 5, severe conjunctival redness with tears and lids more than half closed. Data are presented as boxes and whiskers. The boxes represent the 25th to 75th percentiles and horizontal lines within each box represent the median values of all the data for all time points together. The whiskers are the minimum and the maximum of all the data (n=10; both eyes were evaluated). §P<0.01 versus OVA + vehicle; #P<0.01 versus Ctrl (Friedman test followed by Dunn’s post hoc comparison).
Abbreviations: Ctrl, control; Dex, dexamethasone; Map, mapracorat; OVA, ovalbumin.
