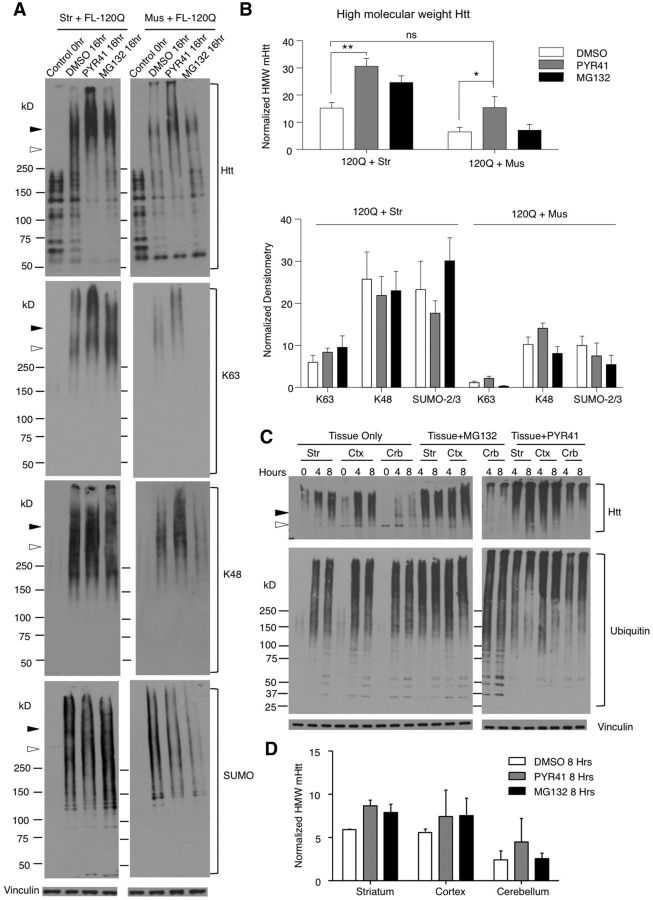
Figure 6.
Promotion of the formation of HMW mHtt and accumulation by inhibiting ubiquitin-activating enzyme E1. A, Western blot is representative of three independent experiments (mEM48 1:50). Striatal tissue lysates (left) or muscle tissue lysates (right) were incubated with transfected full-length mHtt (FL-120Q) for 0 and 16 h with the addition of DMSO, the E1 inhibitor PYR41 (50 μm), or the UPS inhibitor MG132 (50 μm). Open arrowhead, Full-length mHtt; filled arrowhead, mHtt oligomers; Control, lysates before incubation. Blots were probed with (from top to bottom) antibodies to htt (mEM48), α-K63, α-K48, and SUMO-2/3. B, Quantification of HMW mHtt (top), and α-K63, α-K48, and SUMO-2/3 (bottom) at 16 h is shown (n = 3). The ratio of HMW mHtt to vinculin was used for quantification. Error bars indicate the mean ± SEM, and statistical significance was determined using repeated-measures two-way ANOVA with (Tukey's test) multiple testing (*p < 0.05, **p < 0.01). C, HD KI mouse tissue lysates were incubated in vitro in IVDA buffer and treated with the E1 inhibitor PYR41 (50 μm) or the UPS inhibitor MG132 (50 μm). Western blotting showing that PYR41 or MG132 increased the accumulation of oligomerized mHtt in the cerebellar tissue compared with no drug treatment (left). Striatal tissue samples were also included for comparison with peripheral tissues (right). D, Quantification of the ratio of oligomerized mHtt to vinculin after in vitro incubation at 8 h then normalized within the experiment. Error bars indicate the mean ± SEM (n = 3). Str, Striatum; Mus, muscle; Ctx, cortex; Crb, cerebellum.