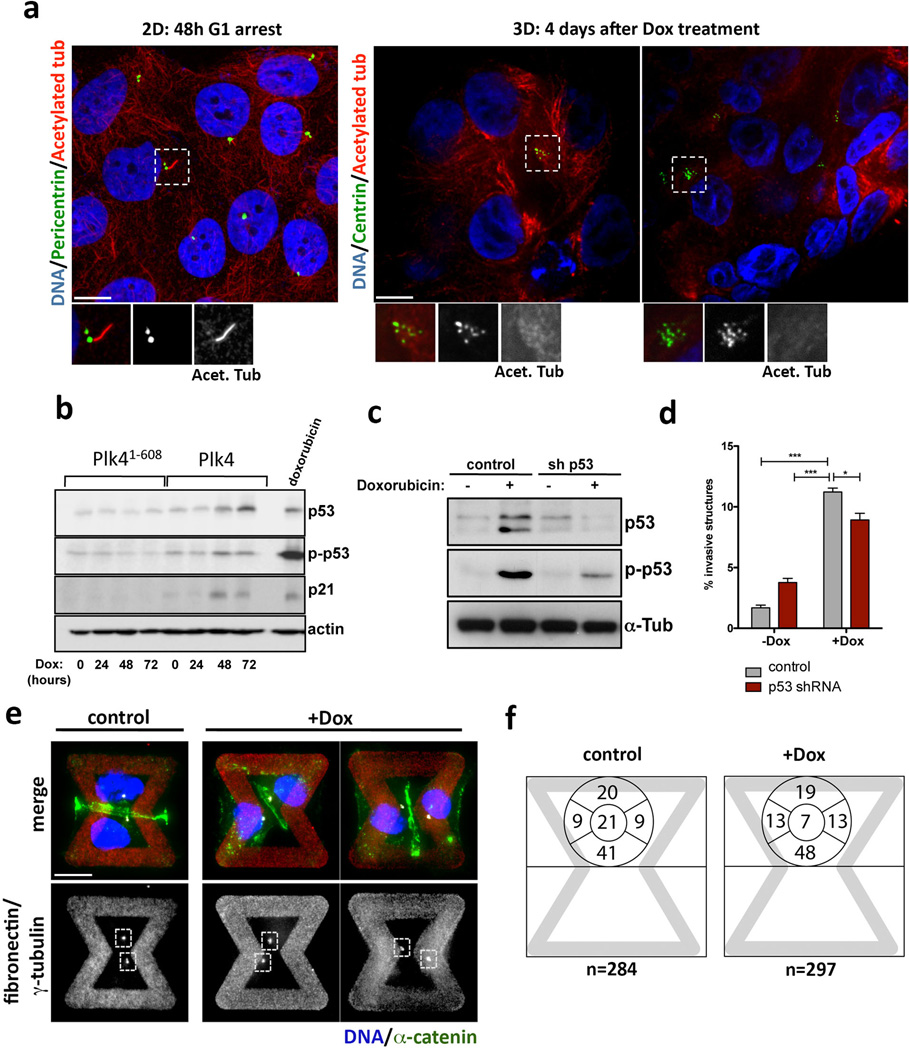
Extended Data Figure 6. Invasive protrusions from 3-D cultures of MCF10A cells with extra centrosomes are not an indirect consequence of altered cilia signaling, increased p53 expression or defects in centrosome polarization.
a, Left: Cells in 2-D were stained for pericentrin (green, inset), acetylated tubulin (red, inset) and DNA (blue). Cells were arrested for 48hrs in G1 to induce primary cilium formation. Note that even in this case most of the cells do not form cilia. This is expected because MCF10A cells have limited proficiency for cilia formation, with only ~7% of the cells assembling cilia even after 7 days of serum starvation43. Right: Cells in 3-D were stained for centrin (GFP, green inset), acetylated tubulin (red, inset) and DNA (blue). Cells do not form cilia after 4 days in 3-D cultures. This is expected because, unlike MDCK cells, MCF10A cells do not have a discernable apical polarity and lumen after 4 days in 3-D cultures and thus unlikely to form primary cilium at this time. b, As expected17, centrosome amplification in MCF10A cells induces modest p53 activation. Note that this degree of p53 activation has a minor impact on the proliferation of MCF10A cells (Fig. S1D). Expression of Plk4 and Plk41–608 was induced by Dox for the indicated times: 0, 24, 48 and 72hrs. c, Western blotting showing the levels of induction of p53 after doxorubicin treatment (200 ng/ml for 4hrs) in control and p53-depleted cells, demonstrating that the p53 shRNA efficiently prevents p53 activation. d, Fraction of acini with invasive protrusions from cells with (+Dox) or without (−Dox) centrosome amplification after depletion of p53. The ability of cells to form invasive acini is not significantly impacted by their p53 status. Error bars represent mean ± SE from 3 independent experiments. p-value derived from unpaired two-tailed t-test (***, p<0.0005; *, p<0.05). e, Cells were stained for α-catenin (green), γ-tubulin (red), DNA (blue) the fibronectin micro-pattern visualized in red. Dashed boxes outline the centrosomes. Note that after Plk4 OE, extra centrosomes (clustered in interphase) are correctly positioned towards the cell-cell junction (similar to the control) even when the junction is defective, suggesting that centrosome amplification is not impairing the polarity axis of these cells. f, Centrosome positioning is not altered in cells with (+Dox) and without (control) extra centrosomes. Left: representative images of cells showing centrosomes (γ-tubulin) in relation to adherens junctions (α-catenin). Right: Scheme with quantification of the fraction of centrosomes at the indicated positions on the micropatterns. (see Fig. 3A). Note that the position of centrosomes in cells with centrosome amplification does not differ from that in control cells. Cells were plated on the patterns 48hrs after induction of centrosome amplification with Plk4.