Abstract
Background:
High-grade primary glioma have poor prognosis and predictive biomarkers is very important. Midkine (MDK), a heparin-binding growth factor, is important in regulating carcinogenesis, cell proliferation, mitogenesis, and angiogenesis. This study aimed to identify over-expression of MDK in gliomas and correlate this with clinical outcomes. The authors put forward their hypothesis correlating proliferation and poor survival with over-expression of this novel protein.
Methods:
Two datasets from Gene Expression Omnibus (GEO) included human data of 100 and 180 patients, respectively. The MDK expression, World Health Organization (WHO) pathological grade, sex, age, and survival time were identified for statistical analysis.
Results:
A search of the GEO profile revealed that MDK expression level was statistically greater in the WHO grade IV compared with grade II (P = 0.002), in grades III and IV compared with nontumor control (P = 0.044 and P < 0.001, respectively) after adjustments using the Bonferroni method. By the Kaplan-Meier survival curve, the high MDK expression group had poorer survival outcome (2.38-fold hazard, 95% confidence interval: 1.22-4.63) than the low MDK expression group after adjustments for WHO grade and age.
Conclusions:
Taken together, there is a positive correlation between MDK expression and WHO grading of human gliomas. Moreover, MDK over-expression is significant correlated to poor survival outcome in high-grade, suggesting that MDK may be an important therapeutic target.
Keywords: GEO profile, glioma, midkine, survival rate, WHO grades
INTRODUCTION
Gliomas are common primary brain tumors with poor outcome despite aggressive interventions.[10] The World Health Organization (WHO) classification of brain tumors established high-grade gliomas with higher mortality.[16] Clinical outcome is often determined by important biomarkers and the identification of genetic expression in glioma is important for improving prognostication.[21]
Midkine (MDK) is a 13-kDa heparin-binding growth factor originally found in embryonal carcinoma cells. The mechanism of MDK action plays as upstream of PI3K and MAPK signaling. MDK receptors included Notch-2, integrins, neuroglycan C, anaplastic lymphoma kinase, low-density lipoprotein receptor-related protein, and receptor protein tyrosine phosphatase Z1 (PTPζ). Thus, MDK activates the PI3K pathway and induces antiapoptotic activity. Moreover, MDK signaling affected some transcription factors, which include Hes-1, STATs, and NF-κB.[18] The over-expression of MDK has been shown in several human malignancies such as oral squamous cell carcinoma,[4] laryngeal carcinoma,[14] breast cancer,[8] hepatoma,[25] renal cell carcinoma,[23] ovarian cancer,[20] cervical cancer,[7] and B-cell lymphomas.[5] Substantial evidence support the role of MDK as an important regulator of carcinogenesis, including cell proliferation, migration-promoting, antiapoptotic, mitogenesis, and angiogenesis.[3,12,17,19] It is also a good and sensitive biomarker that can be detected in serum and used to monitor therapeutic effect in hepatoma.[25]
A recent study using genomic hotspot detection has demonstrated MDK elevation in primary glioma cell lines.[21] However, the role of MDK in clinical outcomes of human glioma is less addressed. This study hypothesized that high-grade gliomas over-express MDK, which determines poor survival outcome. This study aimed to identify over-expression of MDK in gliomas and correlate this with clinical outcomes. The authors put forward their hypothesis correlating proliferation and poor survival with over-expression of this novel protein. Many gene array datasets are deposited in the Gene Expression Omnibus (GEO) profiles that supply online datasets for investigating specific gene expressions that correlate with clinical outcomes.[1,2] This study utilized the GEO datasets through the platform, which we recently established.[24]
MATERIALS AND METHOD
Human MDK gene expression in GEO databases
Two GEO datasets (GDS) were enrolled. The first dataset is GDS1815. The gene array of 100 patients with primary high-grade gliomas showed 100 panels of MDK mRNA expression data in the GEO dataset for analysis [GDS1815/209035_at/MDK]. After excluding 23 panels of data missing age and survival time, 77 panels of data that displayed MDK expression, sex, age, and survival time were enrolled for statistical analysis. The second dataset is GDS1962. The gene array of 180 patients with high-grade gliomas enrolled 180 panels of MDK mRNA expression database (GDS1962/209035_at/MDK). Then, 23 cases of nontumor control, 7 grade II glioma, 19 grade III glioma, and 81 grade IV glioma with the MDK gene expression were reassessed for statistical analysis after excluding 50 panels of oligodendrocytoma data.
Statistical analysis
Categorical variables and continuous variables were presented as number (proportion) and mean ± standard deviation (SD), respectively. Differences in variables between each WHO grade of human glioma samples were compared using the Student's t-test or Chi-square test, as appropriate. The MDK expression was tested by a single tail test based on apparent trend of value in the four WHO grades. The Bonferroni method was used to adjust the P value since multi-groups test had higher possibility of type I error.
The Kaplan-Meier survival curve was used to present the relationships between MDK expression and WHO grades. The relationships were tested using the Cox proportional hazard model. The cut-off point of MDK expression was decided by statistical view as the most significant point in all cut points. Any variable that was significant in univariate analysis was controlled in the multivariate analysis. Statistical significance was set at P < 0.05. All statistical analyses were conducted using the GraphPad Prism 5 software and R 3.0.1 software.
RESULTS
MDK mRNA levels positively correlated with WHO pathological grading of human gliomas
The GEO dataset was statistically analyzed to explore the relationship between WHO pathological grading and MDK expression [Figure 1]. In human gliomas with diverse WHO grades, MDK expression level was statistically greater in WHO grade IV than in grade III (P = 0.020), in grade IV than in grade II (P < 0.001), and in grade IV than in nontumor control (P < 0.001). Differences in MDK expression between grade III and nontumor control (P = 0.007) and between grade II and nontumor control (P = 0.011) were significant, but not between grades II and III (P = 0.229). After adjusting the P value by Bonferroni method, MDK expression was statistically greater in WHO grade IV compared with grade II (P = 0.002), in grade IV compared with nontumor control (P < 0.001), and in grade III compared with nontumor control (P = 0.044).
Figure 1.
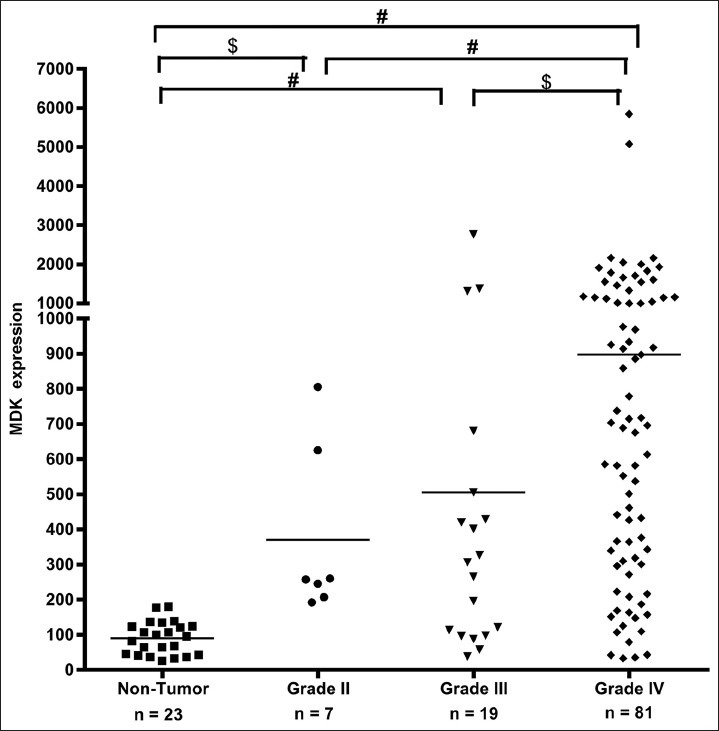
MDK mRNA expression in human gliomas and nontumor control. Scatter plots display the distribution of MDK gene expression in high-grade gliomas (grades III and IV) compared with low-grade glioma and nontumor controls. The WHO grading of gliomas positively correlated with increased mRNA levels of MDK. #adjusted P<0.05; $P<0.05
High MDK mRNA levels positively correlated with poor survival in human gliomas
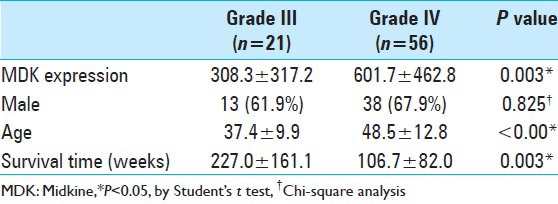
Another GEO dataset (GDS1815/209035_at/MDK) was further analyzed to investigate the correlation among MDK expression, WHO pathologic grades, sex, age, and survival times [Table 1]. The MDK expression level was statistically greater in WHO grade IV compared with grade III (P = 0.003). The survival time of patients with grade IV glioma was shorter than those with grade III glioma (P = 0.003). The mean age of patients with grade III glioma was lower than that of patients with grade IV glioma (P < 0.001).
Table 1.
Statistical analyses of demography in high-grade human gliomas
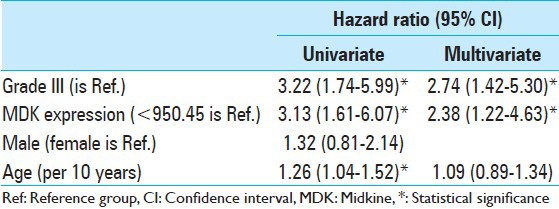
In univariate analysis [Table 2], three variables showed significant association. These were WHO pathologic grade, MDK expression, and age. In multivariate analyses, survival rate was poor in the high MDK expression group (2.38-fold hazard, 95% confidence interval: 1.22-4.63) than in the low MDK expression group after adjustments for WHO grade and age.
Table 2.
Analysis of survival time using Cox proportional hazard model
Using the Kaplan-Meier survival curve, data obtained from the GEO database (GDS1815/209035_at/MDK) was used to explore the correlation between MDK mRNA expression levels and survival of patients with grade III or grade IV gliomas [Figure 2]. The calculated cut-off value was 950.45. Patients with high MDK expression had poorer outcome than those with low MDK expression in high-grade human gliomas (P = 0.0004).
Figure 2.
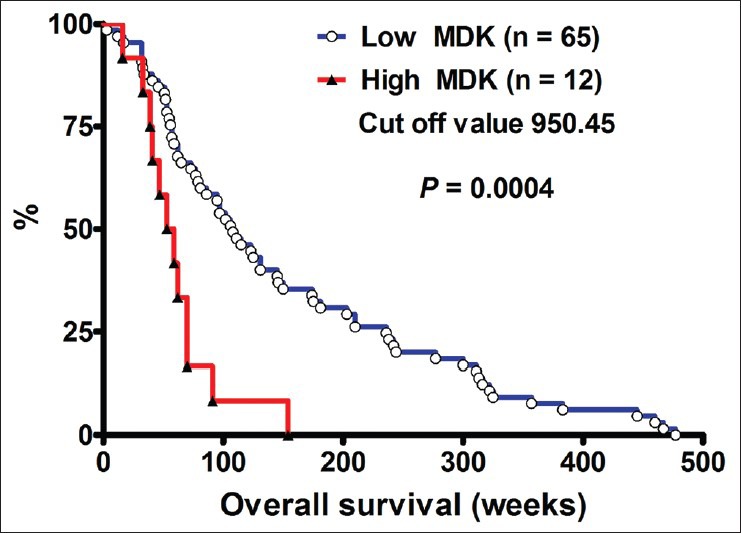
MDK expression level and survival rate of high-grade human gliomas. The Kaplan-Meier survival curve of patients with high MDK expression compared with those with low MDK expression levels (by log-rank test)
DISCUSSION
MDK is a heparin-binding growth factor mediated cellular migration, proliferation, and survival.[22] The mechanism of MDK in glioma is still less addressed. Huang et al. documented that MDK stimulates epithelial-mesenchymal transition (EMT).[6] Their study demonstrated that MDK attached to the Notch2 receptor in HaCaT cells. MDK stimulates Notch2 signaling guiding to protein/protein communications between Jak2/Stat3 and Hes1, suggesting that MDK mediated interaction of Notch2/Jak2/Stat3 signaling pathways and controlled cell motility and tumorigenicity.[6] MDK plays crucial roles in the pathology of cancer, neural disease, and inflammation. The fundamental features of MDK, including genomic organization, protein structure, and physiological functions are well documented by Sakamoto et al.[22]
The present study reveals that the MDK gene expression is positively correlated with WHO grade and poor survival in patients with human gliomas. Similarly, Roversi recognized that MDK gene over-expression correlated with the progression of glioblastoma through genomic array profiling of 25 primary glioma cell lines.[21] Further supportive evidence also shows that MDK has an important role in carcinogenesis and angiogenesis.[3,12,17] Moreover, a previous study has demonstrated that the upregulation of the MDK signaling pathway makes glioma cells refractory to anticancer effects.[15]
MDK is a plasma secreted protein identified in embryonal carcinoma cells at early stages of retinoic acid-induced differentiation.[11] Serum MDK expression is a novel diagnostic marker in the early detection and postoperative follow-up of hepatocellular carcinomas.[25] Moreover, higher plasma MDK concentrations also correlated with poorer outcome in neuroblastomas.[9] Future studies on MDK protein production in serum and cerebrospinal fluid (CSF) may clarify the potential role of MDK in diagnosis and postoperative follow-up. If serum MDK level positively reflects MDK level in CSF, then a simple, low-cost, and relatively noninvasive method can be used to follow-up patients with gliomas.
Nonetheless, this study has some limitations. The MDK expression positively correlates with WHO grading and survival using only a single array data (GDS1815) of human gliomas samples. Thus, the findings and conclusions may not be as robust. Alternatively, another array data (GDS1962) from different medical centers have been analyzed and this confirmed the positive correlation of MDK expression and tumor grading and survival. Importantly, MDK may also have the potential role as a proliferation marker in addition to EGFR/p53 and others that are done for GBMs,[13] which may be used to follow progress of treatment especially if correlated with serum/CSF studies. This study reevaluates the role of MDK in a population consisting of high grade gliomas, low grade and normal brain tissue and correlates survival and grading. Since this factor has been found in highly proliferative brain tumors, it might play a role more as a proliferation marker and add value to Ki67/Mib due to its presence in these tumors. In addition, if found in significant quantities in CSF may prove to have a role in the follow-up and treatment of these patients. Although now data are limited, this point is deserved to be done in this direction.
CONCLUSION
In conclusion, there is a positive correlation between MDK expression and WHO grading of gliomas. In high-grade gliomas, MDK over-expression appears to be significantly correlated to poor survival. Thus, MDK may be a reliable biomarker for determining clinical outcome and pathologic grading. Future studies will further clarify the role of MDK as a possible biomarker for determining clinical outcome and pathologic grading.
ACKNOWLEDGMENTS
This study was supported in part by grants from Tri-Service General Hospital, National Defense Medical Center, (TSGH-C103-005-007-009-S05), the Ministry of Health and Welfare (MOHW103-TD-B-111-12), and National Science Council (NSC 102-2628-B-016 -002 -MY2), Taipei, Taiwan, ROC.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/78/133205
Contributor Information
Yen-Po Cheng, Email: 134309@cch.org.tw.
Chin Lin, Email: xup6fup@hotmail.com.
Ping-Yi Lin, Email: 69221@cch.org.tw.
Chun-Yuan Cheng, Email: 83998@cch.org.tw.
Hsin-I Ma, Email: uf004693@mail2000.com.tw.
Chien-Min Chen, Email: 96015@cch.org.tw.
Dueng-Yuan Hueng, Email: hondy2195@yahoo.com.tw.
REFERENCES
- 1.Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C, et al. NCBI GEO: Archive for high-throughput functional genomic data. Nucleic Acids Res. 2009;37:D885–90. doi: 10.1093/nar/gkn764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: Archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–5. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814–9. [PubMed] [Google Scholar]
- 4.Fujita S, Seki S, Fujiwara M, Ikeda T. Midkine expression correlating with growth activity and tooth morphogenesis in odontogenic tumors. Hum Pathol. 2008;39:694–700. doi: 10.1016/j.humpath.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Hidaka H, Yagasaki H, Takahashi Y, Hama A, Nishio N, Tanaka M, et al. Increased midkine gene expression in childhood B-precursor acute lymphoblastic leukemia. Leuk Res. 2007;31:1045–51. doi: 10.1016/j.leukres.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Hoque MO, Wu F, Trink B, Sidransky D, Ratovitski EA. Midkine induces epithelial-mesenchymal transition through Notch2/Jak2-Stat3 signaling in human keratinocytes. Cell Cycle. 2008;7:1613–22. doi: 10.4161/cc.7.11.5952. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, Sook-Kim M, Ratovitski E. Midkine promotes tetraspanin-integrin interaction and induces FAK-Stat1alpha pathway contributing to migration/invasiveness of human head and neck squamous cell carcinoma cells. Biochem Biophys Res Commun. 2008;377:474–8. doi: 10.1016/j.bbrc.2008.09.138. [DOI] [PubMed] [Google Scholar]
- 8.Ibusuki M, Fujimori H, Yamamoto Y, Ota K, Ueda M, Shinriki S, et al. Midkine in plasma as a novel breast cancer marker. Cancer Sci. 2009;100:1735–9. doi: 10.1111/j.1349-7006.2009.01233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikematsu S, Nakagawara A, Nakamura Y, Sakuma S, Wakai K, Muramatsu T, et al. Correlation of elevated level of blood midkine with poor prognostic factors of human neuroblastomas. Br J Cancer. 2003;88:1522–6. doi: 10.1038/sj.bjc.6600938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 11.Jono H, Ando Y. Midkine: A novel prognostic biomarker for cancer. Cancers (Basel) 2010;2:624–41. doi: 10.3390/cancers2020624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato M, Shinozawa T, Kato S, Awaya A, Terada T. Increased midkine expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2000;124:848–52. doi: 10.5858/2000-124-0848-IMEIHC. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Ishihara K, Shinozawa T, Yamaguchi H, Asano Y, Saito M, et al. Monoclonal antibody to human midkine reveals increased midkine expression in human brain tumors. J Neuropathol Exp Neurol. 1999;58:430–41. doi: 10.1097/00005072-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Xue X, Wang F. The expressions of TP, MK and CD105 in laryngeal squamous cell carcinoma and their clinical significance. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22:100–3. [PubMed] [Google Scholar]
- 15.Lorente M, Torres S, Salazar M, Carracedo A, Hernandez-Tiedra S, Rodriguez-Fornes F, et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011;18:959–73. doi: 10.1038/cdd.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muramatsu T. Midkine and pleiotrophin: Two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem. 2002;132:359–71. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- 18.Muramatsu T. Structure and function of midkine as the basis of its pharmacological effects. Br J Pharmacol. 2014;171:814–26. doi: 10.1111/bph.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muramatsu T, Kadomatsu K. Midkine: An emerging target of drug development for treatment of multiple diseases. Br J Pharmacol. 2014;171:811–3. doi: 10.1111/bph.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rice GE, Edgell TA, Autelitano DJ. Evaluation of midkine and anterior gradient 2 in a multimarker panel for the detection of ovarian cancer. J Exp Clin Cancer Res. 2010;29:62. doi: 10.1186/1756-9966-29-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roversi G, Pfundt R, Moroni RF, Magnani I, van Reijmersdal S, Pollo B, et al. Identification of novel genomic markers related to progression to glioblastoma through genomic profiling of 25 primary glioma cell lines. Oncogene. 2006;25:1571–83. doi: 10.1038/sj.onc.1209177. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto K, Kadomatsu K. Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int. 2012;62:445–55. doi: 10.1111/j.1440-1827.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- 23.Terao S, Shirakawa T, Kubo S, Bishunu A, Lee SJ, Goda K, et al. Midkine promoter-based conditionally replicative adenovirus for targeting midkine-expressing human bladder cancer model. Urology. 2007;70:1009–13. doi: 10.1016/j.urology.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Tsai WC, Chen Y, Huang LC, Lee HS, Ma HI, Huang SM, et al. EMMPRIN expression positively correlates with WHO grades of astrocytomas and meningiomas. J Neurooncol. 2013;114:281–90. doi: 10.1007/s11060-013-1184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu WW, Guo JJ, Guo L, Jia HL, Zhu M, Zhang JB, et al. Evaluation of Midkine as a Diagnostic Serum Biomarker in Hepatocellular Carcinoma. Clin Cancer Res. 2013;19:3944–54. doi: 10.1158/1078-0432.CCR-12-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]


