Abstract
A 10-year old child born to consanguineous parents presented with an extremely large goiter, a low free T4 level and free T4 index, and normal TSH concentration. The findings of undetectable thyroglobulin (TG) and low free T4, and an elevated free T3/free T4 ratio suggested the possibility of a defect in TG synthesis. Noteworthy aspects of this case were the extremely elevated thyroidal radioiodide uptake despite a normal TSH concentration and the fact that the reduction in the size of her goiter only occurred when her TSH was suppressed below the normal range. Gene sequencing revealed that the patient was homozygous for a donor splice site mutation in intron 30 (IVS30 + 1G > C). Isolation of RNA obtained from the thyroid gland by fine needle aspiration and sequencing of the TG cDNA confirmed the prediction that exon 30 was skipped, resulting in an in-frame loss of 46 amino acids.
Keywords: goiter, thyroglobulin deficiency, thyroid disease
Introduction
The genetic defects of thyroid hormone synthesis present most commonly in the neonatal period as congenital hypothyroidism and account for 15%–20% of the newborns with an abnormal thyroid screening test (1). These genetic defects can also present as a diffuse goiter in a euthyroid or mildly hypothyroid child. Molecular testing is usually required for a precise diagnosis in this situation. Nevertheless, a provisional diagnosis can often be made from knowledge of the biosynthetic pathway of thyroid hormone production and application of a few specific tests.
Genetic defects have been described for each of the seven steps in thyroid hormone biosynthesis: (i) The first step involves the active transport of iodide into the thyroid cell against a concentration and electrical gradient, and this process is mediated by a sodium-iodide symporter protein known as NIS. (ii) The second step involves the transport of iodide into the thyroid colloid by the protein pendrin, which is located in the apical membrane of the thyroid follicular cell. Defects in this protein lead to Pendred syndrome. (iii) The template for the synthesis of T4 and T3 is thyroglobulin (TG), which is the most abundant protein in the thyroid gland. It is synthesized in the endoplasmic reticulum of the thyroid cell and secreted into the follicular lumen. Defects in TG synthesis are a relatively common cause of thyroid dyshormonogenesis. (iv) Further processing of the thyroid hormone involves the activity of thyroid peroxidase (TPO), an enzyme also located at the apical membrane of the thyroid follicular cell, and this enzyme deficiency is the most common cause of congenital hypothyroidism resulting from thyroid dyshormonogenesis (2). In the presence of hydrogen peroxide, this enzyme catalyzes iodide organification and the oxidation of I− (iodide) to I2 (iodine), the subsequent iodination of tyrosine residues within the TG molecule, and the coupling of these residues to form the thyroid hormone. (v) Two enzymes have been identified as generators of hydrogen peroxide required for the TPO-catalyzed incorporation of iodine into TG. These are thyroid oxidase or dual oxidase 1 (DUOX1) and dual oxidase 2 (DUOX2). DUOX2 is more highly expressed in the thyroid gland. Deficiency of this enzyme has been described in neonates and adults and may be expressed in a dominant manner (3, 4). (vi) The synthesis of biologically active DUOXs requires the maturation factors DUOXA1 and A2, and the inherited defects in this gene have also been described (5). (vii) The final step in thyroid biosynthesis involves iodotyrosine deiodinase (IYD), an enzyme that deiodinates free, mono- and diiodotyrosines released from TG after its degradation in the phagosomes. The loss of function leads to the inability to recover iodide and its loss in the urine. This often results in iodine deficiency, with the usual clinical picture being goitrous congenital hypothyroidism.
This report describes the diagnostic work-up and genetic analysis of a 10-year-old female who presented with massive thyromegaly, who had a low free T4 and free T4 index and normal TSH, and who was found to have a mutation in the TG gene.
Case report
A 10-year-old girl, originally from Pakistan, was seen for the first time because of massive thyromegaly. She was born to first cousins and had been on thyroid hormone replacement since she was 6 years old. However, this was discontinued 2 years earlier because of lack of health insurance, and her thyroid gland had progressively enlarged. She had no other symptoms. She was midpubertal. Initial laboratory results showed: T4 20.6 mol/L, 57.9–140.3 (1.6 μg/dL, 4.5–10.9), free T4 6.1 pmol/L, 9.1–21 (0.47 ng/dL, 0.71–1.63), free T4 by equilibrium dialysis 7.7 pmol/L, 10.3–34.3 (0.6 ng/dL, 0.8–2.6), total T3 3.6 nmol/L, 0.92–2.8 (2.37 ng/mL, 0.6–1.81), free T3 7.8 pg/mL, 3.5–6.5 (5.05 pg/mL, 2.3–4.2), TSH 2.84 μU/mL, 0.35– 4.00 (2.84 μIU/mL, 0.35– 4.00), thyro globulin 1 μg/L, 2–30 (1 ng/mL, 2–30), T4-binding globulin concentration 0.23 μmol/L, 0.16–0.39 (14 μg/mL, 9.7–23.7), normal thyroxine-binding protein electrophoresis, absent thyroid peroxidase (TPO) and TG antibodies, plasma iodine 236 nmol/L, 316–725 (30 μg/L, 40.1–92.2), and 24-h urine iodine 906 nmol/specimen, 118–3622 (115 μg/specimen, 100–460). A thyroid ultrasound showed a markedly enlarged hypervascular gland with a solid, less vascular nodule measuring 1.3 × 1.6 × 1.3 cm in the lower pole of the left lobe. Because of her low free T4 together with normal TSH, she was worked up for hypopituitarism, and which was negative. She was placed on levothyroxine (L-T4) and a 3-month course of iodine. Over the next 1.5 years, her thyroid gland decreased only slightly in size. A radioactive iodide thyroid scan performed while off L-T4 for 8 weeks showed a 4-h uptake of 94 % (5–15) and a 24-h uptake of 91 % (10–30). The persistent nodule previously detected in the left lower lobe was shown to be a cold nodule. Fine needle aspiration of this nodule was negative for malignancy and revealed reactive follicular cells, colloid, and the presence of hemosiderin-laden macrophages and Hurthle cells suggestive of nodular hyperplasia. An increased dose of Ll-T4 led to suppression of her TSH to 0.13 μU/mL and a significant reduction in goiter size, although her thyroid gland still remained slightly palpable. A repeat radioiodide uptake while off L-T4 for 10 weeks revealed a 4-h uptake of 79 % and a 24-h uptake of 75 %. No discharge of iodine was obtained with use of perchlorate.
Methods
With written consent, blood samples were obtained from the patient and all first-degree relatives for biochemical and genetic studies approved by the Institutional Review Board.
For the values reported in Figure 1A, total T4 and T3 were measured using the commercial automated chemoluminescent immunometric methods and TSH by a third-generation chemiluminescence assay (Elecsys 2010, Roche, Indianapolis, IN, USA). Reverse T3 (rT3) or 3,3′,5-triiodothyronine was measured by radioimmunoassay (Adaltis, Bologna, Italy) and serum TG by an in-house radioimmunoassay with a lower limit of sensitivity of 1.5 pmol/L. The free T4 index (FT4 I) was calculated as the product of the serum total T4 and the normalized resin T4 uptake ratio. TPO and TG antibodies were measured by passive hemagglutination (Fujirebio, Inc., Tokyo, Japan).
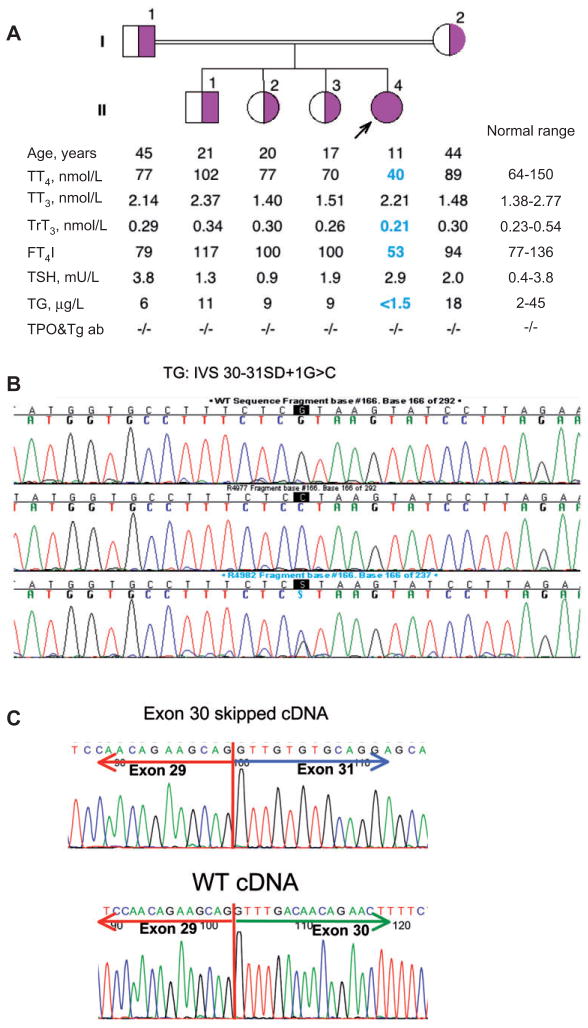
Figure 1.
(A) Results of repeat laboratory testing of proband and close family members when proband was 11 years old. (B) Sequencing of the TG gene revealed a single nucleotide substitution, the normal guanine at the IVS30 splice donor site being replaced by a cytosine. (C) Sequencing of the TG synthesized by reverse transcription of mRNA from the thyroid gland confirmed the predicted skipping of exon 30.
Genomic DNA was extracted from circulating mononuclear cells, and all TG exons were amplified by the polymerase chain reaction (PCR) and sequenced. The PCR conditions will be provided upon request. The thyroid tissue obtained during fine needle aspiration was immediately placed in TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and shipped by FedEx from Allentown to Chicago. The total RNA was extracted, and the first strand of cDNA was synthesized using the SuperScriptTM III First-Strand Synthesis System for reverse transcriptase (RT)-PCR protocol (Invitrogen Life Technologies, Carlsbad, CA, USA). TG cDNA was then amplified by PCR using specific intronic primer pairs and sequenced.
Results
The results of repeat laboratory testing at 11 years of age are shown in Figure 1A. The proposita showed a low-serum total T4 and FT4I concentrations as well as low reverse T3, while her T3 and TSH remained in the normal range. Her TG concentration was undetectable. Her three older siblings and parents had no thyroid test abnormalities (Figure 1A).
The sequencing of the TG gene using genomic DNA extracted from the circulating leukocytes revealed a single nucleotide substitution, the normal guanine at the IVS30 splice donor site being replaced by a cytosine, changing the GT signal to CT. The proband was homozygous for the mutation, while both parents and three siblings were heterozygous (Figure 1B).
As shown in Figure 1C, the sequence of TG synthesized by reverse transcription on the mRNA obtained by fine needle aspiration of the thyroid gland confirmed the predicted skipping of exon 30.
Discussion
The usual presentation of a TG gene defect is severe hypothyroidism with a goiter being present at birth or shortly thereafter (6). However, a later presentation with a goiter in childhood is well recognized. Some of these patients had normal TSH levels and presented with a large goiter in childhood or adulthood, as did this patient (7–9).
The newly synthesized monomeric TG forms dimers within the thyroid follicles (10). The TG molecule needs to be adequately folded to successfully migrate into the colloid, and the mutations that impede the export of the mutant TG lead to a form of endoplasmic reticulum storage disease (11). The follicular cells appear tall or cuboidal and contain yellow-green granules, while the thyroid follicles remain small and depleted of colloid (12). The euthyroid state can be explained by the mutant TG escaping from the endoplasmic reticulum to undergo iodination in the colloid (13, 14).
The blood levels of TG in this condition are usually undetectable or low, in contrast to the normal or elevated levels in other forms of thyroid dyshormonogenesis. Also typical is a low level of T4 in combination with an elevated T3, resulting in an elevated T3/T4 ratio, and this is probably due to an increased thyroidal type 2 iodothyronine deiodinase activity (15). Radioiodide uptake may be normal or increased, and perchlorate discharge is usually normal (16).
TG deficiency in this patient was suggested by the low levels of TG and free T4 and elevated free T3/free T4 ratio. The radionucleotide uptake was markedly increased and in excess of what might have been expected with euthyroid status. For this reason, the thyroidal radioiodide uptake was repeated some years later after 10 weeks without L-T4 replacement, and similar results were obtained. Increased radioiodide uptake has been noted in other cases and associated with both high and normal serum TSH concentrations (14, 17).
It is suggested that increased levels of free T3 maintained this patient in a clinically euthyroid state. The increase in radionucleotide uptake may have been due to a high turnover of abnormal TG, although this is speculative. Of interest is that replacement L-T4 led to only a slight reduction in goiter size when the patient’s TSH level was kept within the normal range. However, a considerable diminution in size occurred when her TSH was suppressed below the normal range (18).
The development of thyroid cancer has been described for several forms of thyroid dyshormonogenesis and may be a particular concern in euthyroid and goitrous TG deficiency (19, 20). Hishinuma et al. (20) reported thyroid malignancy in seven of 11 adults with Tg deficiency with surgically removed goiters. Thyroid supplementation appears to have been ineffective in preventing the development of cancer in some of these patients. Medeiros-Neto and Stanbury (21) found 12 cases of malignancy in approximately 106 pathological examinations of patients with congenital goiter and noted that most of these patients had been submitted to near-total thyroidectomies when young and may have had chronically elevated TSH values. Our patient did show a persistent “cold” thyroid nodule on radioiodide scanning, and it was thought appropriate, therefore, to perform a fine needle aspiration, which revealed nodular hyperplasia.
The TG molecule is encoded by a large gene located on chromosome 8q2. It spans more than a 300-kb region and contains 48 exons. About 50 mutations have been described in the TG gene, including non-sense and missense mutations, single nucleotide insertions and deletions, large nucleotide deletions, and acceptor and donor splice site mutations (22, 23). Genomic analysis of this patient revealed a donor splice site mutation in the first nucleotide of intron 30 (IVS30 + 1G > C), leading to exon skipping and, as a consequence, the in-frame loss of 46 amino acids. The correct splicing of the TG gene at this location requires a nucleotide sequence of GT, but the replacement of the normal guanine with a cytosine produced the sequence CT. To confirm the predicted skip of exon 30, thyroid cell material obtained during fine needle aspiration of the patient’s thyroid served as a source of RNA. Complementary DNA was synthesized and sequenced, thereby confirming the predicted skipping of exon 30 and in-frame loss of 46 amino acids.
This precise nucleotide substitution has not previously been described, although other donor splice site mutation defects resulting in the skipping of exon 30 have been reported, and these reveal variability in the expression of the defect. Pardo et al. (13, 24) and Targovnic et al. (25, 26) described the sibling pairs with congenital hypothyroidism and the genetic defect IVS30 + 1G > T, also leading to loss of the entire exon 30. Hishinuma et al. (16) reported 52 patients with TG deficiency, including one patient with IVS30 + 1G > A in whom the normal guanine was replaced by adenine. Details of the individual patients were not provided, but the symptoms were described as mild, and longstanding goiters were the only clinical manifestations in these euthyroid or mildly hypothyroid adults.
In conclusion, a clinically euthyroid child is described who presented with a large goiter and normal TSH level. The laboratory features suggested a TG gene defect, namely, the undetectable TG and low free T4 levels and elevated free T4/free T4 ratio. She also had an extremely elevated radioiodide uptake. The diagnosis of a TG deficiency was confirmed by genetic analysis, which showed a donor splice site mutation in the first nucleotide of intron 30 (IVS30 + 1G > C). The resulting skipping of exon 30 was confirmed by sequencing mRNA obtained by needle aspiration of the thyroid.
Acknowledgments
This work was supported, in part, by Grants DK15070, DK20595, and RR04999 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Contributor Information
Pia Hermanns, Pediatric Endocrinology, Johannes Gutenberg Children’s Hospital, Mainz, Germany.
Samuel Refetoff, Medicine, The University of Chicago, Chicago, IL, USA; and Pediatrics and Genetics, The University of Chicago, Chicago, IL, USA.
Chutintorn Sriphrapradang, Medicine, The University of Chicago, Chicago, IL, USA.
Joachim Pohlenz, Pediatric Endocrinology, Johannes Gutenberg Children’s Hospital, Mainz, Germany.
Jessica Okamato, Pediatric Endocrinology, Lehigh Valley Hospital, Allentown, PA, USA.
Leeyat Slyper, Pediatric Endocrinology, Lehigh Valley Hospital, Allentown, PA, USA.
Arnold H. Slyper, Email: arnold_h.slyper@lvh.com, Professor of Pediatrics, 400 N 17th Street, Suite 201, Allentown, PA 18104, USA, Phone: +484 664 7850
References
- 1.Grasberger H, Refetoff S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr. 2011;23:421–8. doi: 10.1097/MOP.0b013e32834726a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park SM, Chatterjee VK. Genetics of congenital hypothyroidism. J Med Genet. 2005;42:379–89. doi: 10.1136/jmg.2004.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010;322:99–106. doi: 10.1016/j.mce.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Ohye H, Fukata S, Hishinuma A, Kudo T, Nishihara E, et al. A novel homozygous missense mutation of the dual oxidase 2 (DUOX2) gene in an adult patient with a large goiter. Thyroid. 2008;18:561–6. doi: 10.1089/thy.2007.0258. [DOI] [PubMed] [Google Scholar]
- 5.Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, et al. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypo thyroidism. J Clin Endocrinol Metab. 2008;93:605–10. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medeiros-Neto G, Targovnik HM, Vassart G. Defective thyroglobulin synthesis and secretion causing goiter and hypothyroidism. Endocr Rev. 1993;14:165–83. doi: 10.1210/edrv-14-2-165. [DOI] [PubMed] [Google Scholar]
- 7.Corral J, Perez R, Martin C, Sanchez I, Mories MT, et al. Thyroglobulin gene point mutation associated with non-endemic simple goiter. Lancet. 1993;341:462–4. doi: 10.1016/0140-6736(93)90209-y. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Centeno C, Gonzalez-Sarmiento R, Mories MT, Corrales JJ, Miralles-Garcia JM. Thyroglobulin exon 10 gene point mutation in a patient with endemic goiter. Thyroid. 1996;6:423–7. doi: 10.1089/thy.1996.6.423. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Sarmiento R, Corral J, Mories MT, Corrales JJ, Miguel-Velado E, et al. Monoallelic deletion in the 5′ region of the thyroglobulin gene as a cause of sporadic nonendemic simple goiter. Thyroid. 2001;11:789–93. doi: 10.1089/10507250152484655. [DOI] [PubMed] [Google Scholar]
- 10.Kim PS, Kim K-R, Arvan P. Disulfide-linked aggregation of thyroglobulin normally occurs during nascent protein folding. Am J Physiol. 1993;265:C704–11. doi: 10.1152/ajpcell.1993.265.3.C704. [DOI] [PubMed] [Google Scholar]
- 11.Vono-Toniolo J, Rivolta CM, Targovnik HM, Medeiros-Neto G, Kopp P. Naturally occurring mutations in the thyroglobulin gene. Thyroid. 2005;15:1021–33. doi: 10.1089/thy.2005.15.1021. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Takamatsu J, Kuma K, Murakami Y, Sakane S, et al. A variant of adenomatous goiter with characteristic histology and possible hereditary thyroglobulin abnormality. J Clin Endocrinol Metab. 1996;81:961–1966. doi: 10.1210/jcem.81.5.8626865. [DOI] [PubMed] [Google Scholar]
- 13.Pardo V, Vono-Toniolo J, Rubio IG, Knobel M, Possato RF, et al. The p. A2215D thyroglobulin gene mutation leads to deficient synthesis and secretion of the mutated protein and congenital hypothyroidism with wide phenotype variation. J Clin Endocrinol Metab. 2009;94:2938–44. doi: 10.1210/jc.2009-0150. [DOI] [PubMed] [Google Scholar]
- 14.Hishinuma A, Kasai K, Masawa N, Kanno Y, Arimura M, et al. Missense mutation (C1263R) in the thyroglobulin gene causes congenital goiter with mild hypothyroidism by impaired intracellular transport. Endocr J. 1998;45:315–27. doi: 10.1507/endocrj.45.315. [DOI] [PubMed] [Google Scholar]
- 15.Kanou Y, Hishinuma A, Tsunekawa K, Seki K, Mizuno Y, et al. Thyroglobulin gene mutations producing defective intracellular transport of thyroglobulin are associated with increased thyroidal type 2 iodothyronine deiodinase activity. J Clin Endocrinol Metab. 2007;92:1451–7. doi: 10.1210/jc.2006-1242. [DOI] [PubMed] [Google Scholar]
- 16.Hishinuma A, Fukata S, Nishiyama S, Nishi Y, Oh-Ishi M, et al. Haplotype analysis reveals founder effects of thyroglobulin gene mutations C1058R and C1977S in Japan. J Clin Endocrinol Metab. 2006;91:3100–4. doi: 10.1210/jc.2005-2702. [DOI] [PubMed] [Google Scholar]
- 17.Raef H, Al-Rijjal R, Al-Shehri S, Zou M, Al-Mana H, et al. Biallelic p. R2223H mutation in the thyroglobulin gene causes thyroglobulin retention and severe hypothyroidism with subsequent development of thyroid carcinoma. J Clin Endocrinol Metab. 2010;95:1000–6. doi: 10.1210/jc.2009-1823. [DOI] [PubMed] [Google Scholar]
- 18.Anselmo J, Refetoff S. Case history. Regression of a large goiter in a patient with resistance to thyroid hormone by every other day treatment with triiodothyronine. Thyroid. 2004;14:71–4. doi: 10.1089/105072504322783876. [DOI] [PubMed] [Google Scholar]
- 19.Alzahrani AS, Baitei EY, Zou M, Shi Y. Metastatic follicular thyroid carcinoma arising from congenital goiter as a result of a novel splice donor site mutation in the thyroglobulin gene. J Clin Endocrinol Metab. 2006;91:740–6. doi: 10.1210/jc.2005-2302. [DOI] [PubMed] [Google Scholar]
- 20.Hishinuma A, Fukata S, Kakudo K, Murata Y, Ieiri T. High Incidence of thyroid cancer in long-standing goiters with thyroglobulin mutations. Thyroid. 2005;15:1079–84. doi: 10.1089/thy.2005.15.1079. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros-Neto G, Stanbury JB. Thyroid malignancy and dyshormonogenetic goiter. In: Medeiros-Neto G, Stanbury JB, editors. Inherited disorders of the thyroid system. Boca Raton: CRC Press; 1994. pp. 207–18. [Google Scholar]
- 22.Rivolta CM, Targovnik HM. Molecular advances in thyroglobulin disorders. Clin Chim Acta. 2006;374:8–24. doi: 10.1016/j.cca.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Targovnik HM, Esperante SA, Rivolta CM. Genetics and phenomics of hypothyroidism and goiter due to thyroglobulin mutations. Mol Cell Endocrinol. 2010;322:44–55. doi: 10.1016/j.mce.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Pardo V, Rubio IG, Knobel M, Aguiar-Oliveira MH, Santos MM, et al. Phenotypic variation among four family members with congenital hypothyroidism caused by two distinct thyroglobulin gene mutations. Thyroid. 2008;18:783–6. doi: 10.1089/thy.2007.0321. [DOI] [PubMed] [Google Scholar]
- 25.Targovnik HM, Vono J, Billerbeck AE, Cerrone GE, Varela V, et al. A 138-nucleotide deletion in the thyroglobulin ribonucleic acid messenger in a congenital goiter with defective thyroglobulin synthesis. J Clin Endocrinol Metab. 1995;80:3356–60. doi: 10.1210/jcem.80.11.7593451. [DOI] [PubMed] [Google Scholar]
- 26.Targovnik HM, Rivolta CM, Mendive FM, Moya CM, Vono J, et al. Congenital goiter with hypothyroidism caused by a 5′ splice site mutation in the thyroglobulin gene. Thyroid. 2001;11:685–90. doi: 10.1089/105072501750362763. [DOI] [PubMed] [Google Scholar]