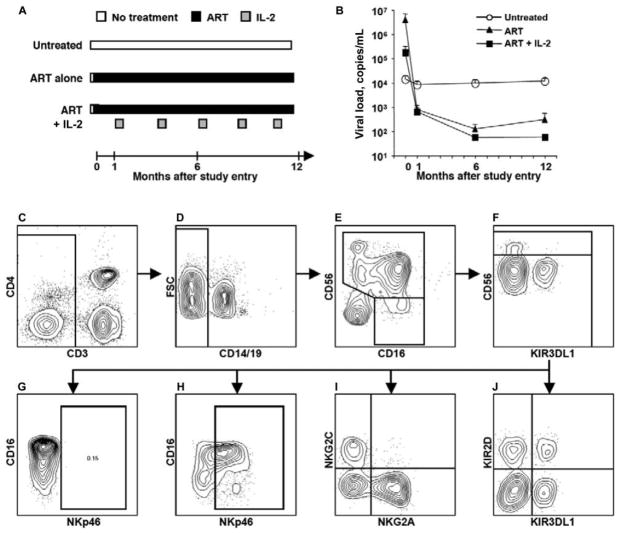
Figure 1.
Study design and gating strategy for flow cytometry. A, The 3 groups of HIV-1–infected patients examined. All 3 groups were treatment naive at study entry (month 0), when the first sample was collected. The first group elected not to initiate antiretroviral therapy (ART) and remained untreated throughout the study (untreated; n = 11). Note that this group was not randomized to remain untreated and is thus likely biased in relation to the 2 treated groups. The 2 other groups initiated ART (ART; n = 11) at study entry and were randomized to either continue receiving ART during the first year (ART alone) or to initiate interleukin (IL)–2 therapy once their viral load was <500 copies/mL (ART + IL-2). All subjects in these groups responded to ART. IL-2 was given twice daily for 5 days in 8-week intervals. Samples at week 24 and 48 were analyzed on average 6 weeks after the last cycle of IL-2 administration and on average 14 weeks after the first dose of IL-2. B, Mean viral load over time in subjects who remained untreated throughout the study (white circles), in subjects who initiated ART at study entry (black triangles), and in subjects who initiated ART at study entry, followed by IL-2 therapy (black squares). Error bars indicate SEs. Note that the viral load of the untreated group is significantly lower than those of the treated groups at study entry. C–J, Gating strategy for phenotyping of NK cells from HIV-1–infected individuals by use of 8-color flow cytometry. After gating on lymphocytes using forward and side light scatter (FSC and SSC, respectively), doublet cells were excluded using an FSC-area vs. FSC-height gate (data not shown). NK cells were defined by gating on CD3− cells (C), followed by exclusion of B cells and monocytes based on the expression of CD19 and CD14 (D), respectively. CD56+ NK cells were defined by a CD56+ gate among the CD3−CD14−CD19− cells (E). A small subset of cells, previously described as dysfunctional NK cells, were defined by gating on CD56−CD16+ cells among the CD3−CD14−CD19− cells. To define NK cells within this gate, CD11cbrightCD4low cells among the CD3−CD56−CD16+ cells were excluded (data not shown). CD56+ NK cells were subsequently split into CD56bright and CD56dim NK cells, based on the expression level of CD56 (F). Fluorescence minus one (FMO) controls were used to define the background fluorescence for each fluorochrome (G). An example of an FMO control for allophycocyanin-conjugated anti-NKp46 is shown. CD56dim NK cells were further phenotyped for NK cell receptor expression by use of combinations of different anti–NK cell receptor antibodies, including anti-NKp46 (H), anti-NKG2A and anti-NKG2C (I), and anti-KIR3DL1 and anti-KIR2D (J).