Figure 2.
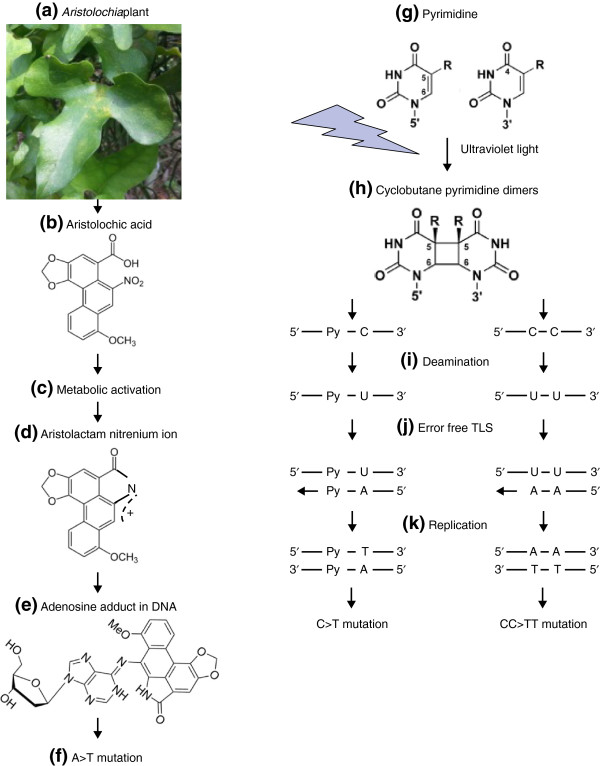
Mechanisms of mutagenesis of aristolochic acid and UV light. Preparations of (a) plants from the genus Aristolochia contain (b) aristolochic acid. Aristolochic acid I is shown; in aristolochic acid II, OCH3 is replaced by H. Aristolochic acid is (c) metabolically activated to (d) aristolactam nitrenium ions by one or more of several enzymes, including NQO1 (NAD(P)H dehydrogenase, quinone 1), CYP1A2 (cytochrome P450, family 1, subfamily A, polypeptide 2), and NADPH-hemoprotein reductase [45]. (e) The aristolactam nitrenium ions form covalent adducts with adenosine bases, and (f) these adducts lead to A > T mutations. (g) Pyrimidines exposed to ultraviolet (UV) radiation form (h) cyclobutane pyrimidine dimers (CPD). (i) Either the cytosine (C) (left) or the CC dipyrimidines in CPD (right) undergo deamination, resulting in uracil (U). Py denotes pyrimidine. (j) Error-free trans-lesion DNA synthesis (TLS) induces C > T and CC > TT mutations at the sites of U-containing CPDs through DNA replication of the U-containing DNA strand (k). Photograph of Aristolochia plant (a) by ST Pang. Molecule schematics in (b-e) reproduced from [14], with permission from Oxford University Press.
