Abstract
Endoscopic ultrasound (EUS) is a reference technique for diagnosing and staging several different diseases. EUS-guided biopsies and fine needle aspirations are used to improve diagnostic performance of cases where a definitive diagnosis cannot be obtained through conventional EUS. However, EUS-guided tissue sampling requires experience and is associated with a low but not negligible risk of complications. EUS elastography is a non-invasive method that can be used in combination with conventional EUS and has the potential for improving the diagnostic accuracy and reducing the need for EUS-guided tissue sampling in several situations. Elastography measures tissue stiffness by evaluating changes in the EUS image before and after the application of slight pressure to the target tissue by the ultrasonography probe. Pathologic processes such as cancerization and fibrosis alter tissue elasticity and therefore induce changes in elastographic appearance. Qualitative elastography depicts tissue stiffness using different colors, whereas quantitative elastography renders numerical results expressed as a strain ratio or hue histogram mean. EUS elastography has been proven to differentiate between benign and malignant solid pancreatic masses, as well as between benign and malignant lymph nodes with a high accuracy. Studies have also demonstrated that the early changes of chronic pancreatitis can be distinguished from normal pancreatic tissues under EUS elastography. In this article, we review the technical aspects and current clinical applications of qualitative and quantitative EUS elastography and emphasize the potential additional indications that need to be evaluated in future clinical studies.
Keywords: endoscopic ultrasound, elastography, pancreatic tumors, lymph nodes
Introduction
The introduction of endoscopic ultrasound (EUS) in clinical practice has introduced an important advancement in the management of a wide variety of diseases. EUS has been demonstrated to have significantly changed the diagnosis and/or management of up to 25% to 50% of cases.1,2,3,4,5,6,7 Nevertheless, an accurate diagnosis cannot always be determined using only conventional B-mode EUS imaging. In many cases, EUS-guided fine needle aspiration (FNA) and/or biopsy can provide a definitive diagnosis. The accuracy of EUS-guided FNA has been demonstrated to be very high, with sensitivity between 80% and 85%, and specificity approaching 100%.8,9,10,11,12,13,14 However, EUS-guided tissue sampling is technically demanding, and multiple punctures may be necessary to obtain a sufficient amount of tissue.15,16 Furthermore, despite repeated sampling, cytohistologic assessment can be falsely negative, which is reported most frequently for solid pancreatic masses in patients with advanced chronic pancreatitis (CP).17 Another limitation of conventional EUS is related to lymph node evaluation. Although EUS provides highly accurate images of lymph nodes, the differentiation of benign from malignant lymph node enlargement can be difficult. The established features of malignancy during lymph node evaluation are a round shape, hypoechoic structure, diameter >1 cm, and distinct margins. However, the specificity of malignancy detection using these criteria is still poor.18 EUS-guided FNA overcomes this problem. However, several enlarged lymph nodes are often present in a patient and conventional EUS provides little help in identifying the optimal node to biopsy. Furthermore, EUS-guided FNA is associated with small, but not insignificant morbidity rates.19,20 Hence, new methods have been warranted, allowing for a more accurate but still non-invasive characterization of lesions, limiting the need for EUS-guided tissue sampling and guided biopsies of areas with the highest suspicion of malignant in cases where tissue sampling is still necessary. To this end, new techniques such as contrast-enhanced EUS and EUS elastography have emerged. Contrast-enhanced EUS has recently been reviewed elsewhere21,22 and will not be covered in this article.
Elastography is a real-time method for evaluation of tissue stiffness. Several different pathologies, including cancer, can induce alterations in tissue stiffness. Elastography was initially developed for evaluation of lesions accessible from the body surface.23,24 Today, elastographic evaluation can be performed from the inside the gastrointestinal tract combined with conventional EUS. Promising results have been reported for EUS elastography in several studies, indicating its high accuracy in differentiating benign from malignant lesions both in the pancreas and in lymph nodes. The aim of the present article is to review the technical aspects and clinical applications of EUS elastography and to identify related fields for further studies.
Technical aspects and method of elastography
Elastography can be regarded as a development from the well-known fremitus technique in breast ultrasonography, which demonstrates that healthy breast tissue vibrate more than solid malignant lesions, despite their isoechoic appearance under B-mode ultrasound.25,26,27,28,29 The basis for elastography is the fact that many different pathologic processes, including inflammation, fibrosis, and cancer, induces alterations in tissue stiffness. Elastography evaluates tissue stiffness through the application of slight compression using an ultrasound transducer to the targeted tissue and recording the resulting tissue displacement in the examined field.30,31 Elastography is performed and evaluated in real time using a conventional EUS probe attached to a processor with specific software installed. Physiologic vascular pulsations and respiratory movements provide the vibrations and compressions necessary for the recording. The first generation of EUS elastography allowed only qualitative evaluation. Today, sthe second generation also allows quantitative evaluation of tissue stiffness.26,32
Qualitative EUS-Elastography
Qualitative elastography relies on the quantification of the compression-induced deformation of the structures in the B-mode image using the degree of deformation as an indicator of tissue stiffness.23,33 Elasticity (on a scale of 1 to 255) is depicted using a color map (red-green-blue), wherein hard tissue is shown in dark blue, medium hard tissue in cyan, tissue with intermediate hardness in green, medium soft tissue in yellow, and soft tissue in red. Elastography pattern is demonstrated by superimposing the color pattern on a conventional B-mode picture. Usually, a two-panel image is used for presentation, with the conventional grey-scale B-mode image on the right side and the elastographic image on the left (Figure 1).
Figure 1.

Qualitative EUS elastography of a pancreatic cancer showing a specific color distribution
For the recording, the EUS probe was pressed onto the gut wall with the same pressure needed to generate an optimal and stable B-mode image at 7.5 MHz. The region of interest (ROI) for the elastographic evaluation was manually selected. Optimally, the ROI includes the whole target lesion, as well as some surrounding tissues for reference. An image stable for at least five seconds is required for the final color pattern characterization because the colors can fluctuate.34
Quantitative EUS-Elastography
There are two options for quantitative elastography evaluation, a hue histogram and strain ratio calculation.
Hue Histogram
The hue histogram is a graphical representation of the color distribution (hues) in a selected image field. Hue histograms are based on the qualitative EUS elastography data for a manually selected ROI within the standard elastography image. The x-axis in the hue histogram represents the elasticity from 0 (softest) to 255 (hardest) of the tissue. The y-axis represents the number of pixels in each elasticity level in the ROI. The mean value of the histogram corresponds to the global hardness or elasticity of the lesion.35 Software that constructs and analyzes hue histograms from EUS elastography images is readily available (Image J software, NIH, Bethesda, MD, USA).36 All recent Hitachi platforms have integrated software for hue histogram analysis. This software also uses a hue scale from 0 to 250, but unlike Image J, 0 represents the hardest and 255 is the softest (Figure 2).
Figure 2.
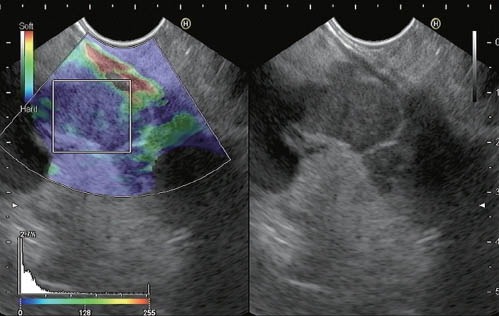
Quantitative EUS elastography based on hue histogram analysis of a metastatic intraabdominal lymph node (gastric adenocarcinoma). The histogram analysis was performed on a selected area within the region of interest. The mean value of this evaluation is shown at the bottom of the image (14.5).
Strain Ratio
Qualitative elastography patterns are relative to some extent. The calculation of strain ratio, which analyzes the elastographic picture of the target lesion in relation to the surrounding tissues, is an attempt to address this problem.37 Similar to the hue histogram, strain ratio calculation is based on standard qualitative EUS elastography data. Two different areas (A and B) are selected for quantitative elastographic analysis. Area A is selected so that it includes as much of the target lesion as possible without including the surrounding tissues. Area B is selected within a soft (red) reference area outside the target lesion, preferably the gut wall. The strain ratio is calculated as the quotient of B/A38 (Figure 3). A presumption of this method is that the investigated disease does not significantly alter the hardness of the reference connective or fat tissues.
Figure 3.
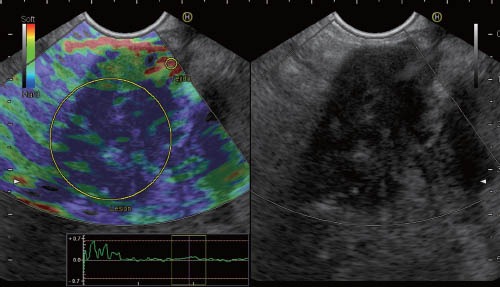
Quantitative EUS elastography based on strain ratio analysis of a solid pancreatic mass (pancreatic adenocarcinoma). We selected area A to represent pancreatic parenchyma, and area B to correspond to a soft area from the gut wall. The B/A ratio is displayed at the bottom of the image (55.66).
Clinical applications of EUS-elastography
The evaluation of solid pancreatic lesions and enlarged lymph nodes are the two main indications for EUS elastography. However, some additional indications may increase in importance in the near future.
Pancreatic diseases
Today, EUS is considered a reference method for the diagnosis and staging of inflammatory, cystic, and neoplastic lesions of the pancreas.4,39,40 Nevertheless, the capability of conventional B-mode EUS to differentiate between benign and malignant pancreatic lesions can be less than optimal in some clinical situations.4 For example, its overall accuracy in differentiating between pancreatic cancer and focal pancreatitis in advanced chronic pancreatitis is not higher than 75%.41 EUS elastography may be a useful tool in these situations.
Differential diagnosis of solid pancreatic lesions
The first study of EUS elastography in pancreatic solid lesions was published by Giovannini et al.42. A total of 24 pancreatic masses were analyzed using a subjective scoring system based on the different color patterns of the images. The lesions that appear mainly blue (harder) were classified as malignancies. Based on this classification, the sensitivity and specificity of the malignancy detection was 100% and 67%, respectively. In this paper, we also established a more refined classification for elastographic appearance as follows: A score of 1 was defined as homogeneous soft tissue (green) and interpreted as normal tissue. A score of 2 was given to heterogeneous soft tissue (green, yellow, and red), and interpreted as fibrosis or inflammation. A score of 3 represents mixed hard and soft tissues (mixed colors) or a honeycombed elastography pattern, interpreted as indeterminate for malignancy. A score of 4 was given for hard (blue) lesions with a soft (green) central area, interpreted as malignant, hypervascularized lesions. Finally, a score of 5 represents predominantly hard (blue) lesions with dispersed heterogenic soft (green, red) areas, interpreted as advanced malignant lesions with necrotic areas.42 In a subsequent multicenter trial, Giovanni et al. reported EUS elastography findings in 121 cases with pancreatic masses.43 They used the classification they previously made, classifying scores of 1 and 2 as benign, and 3 to 5 as malignant. The sensitivity, specificity, positive predictive value, and negative predictive value of the differentiation between benign and malignant pancreatic masses were 92.3%, 80.0%, 93.3%, and 77.4%, respectively, and an overall accuracy of 89.2%. The interobserver agreement of the evaluation of 30 cases yielded a kappa score of 0.785 (substantial agreement according to Landis and Koch)44 in the detection of malignancy. We have published our own experience with qualitative EUS elastography in 130 patients with solid pancreatic masses and 20 controls. Four different patterns, similar to those described by Giovannini et al. were detected as follows: a homogeneous green pattern present only in normal pancreas; a heterogeneous, predominantly green pattern with slight yellow and red lines present only in inflammatory pancreatic masses; a heterogeneous, predominantly blue pattern with small green areas and red lines, and a geographic appearance, present mainly in pancreatic malignant tumors (including pancreatic adenocarcinoma); and a homogeneous blue pattern, present only in pancreatic neuroendocrine malignant lesions. Using this classification, the sensitivity, specificity, positive and negative predictive values, and overall accuracy of EUS elastography for detecting malignancy were 100%, 85.5%, 90.7%, 100%, and 94.0%, respectively. All of the patients were evaluated by two endosonographers who made the same interpretation in 121/130 cases and 20/20 controls, yielding a kappa value of 0.772.34
Not all studies have observed this level of accuracy in EUS elastography for differentiating between benign and malignant pancreatic lesions. Jansen et al.45 investigated 20 patients with normal pancreas, 20 with CP, and 33 with focal pancreatic lesions using qualitative EUS elastography and obtained a similar sensitivity (93.8%), but clearly an inferior specificity (65.4%) compared with the aforementioned studies. Their overall accuracy for malignancy detection was 73.5%. Hirche et al.46 also published results of the qualitative EUS elastography of 70 patients with unclassified solid pancreatic lesions and 10 controls. In their study, adequate elastographic evaluation was obtained in only 56% of the patients. The study pointed out some clinical situations where adequate elastography evaluation may be difficult, including difficulties in including an entire lesion and enough surrounding tissues in the analyzed ROI in large (>35 mm) lesions, lesions distant from the transducer, and presence of fluid (vessels, cysts, etc.) in the ROI. Overall, EUS elastography predicted the nature of pancreatic lesions with poor diagnostic sensitivity (41%), specificity (53%), and accuracy (45%).
More recent studies have analyzed the usefulness of quantitative EUS-elastography. We have published the strain ratio results of 86 consecutive patients with pancreatic solid lesions (49 adenocarcinomas, 27 inflammatory masses, 6 malignant neuroendocrine tumors, 2 metastatic oat cell lung cancers, 1 pancreatic lymphoma, and 1 pancreatic solid pseudopapillary tumor) and 20 controls. The strain ratio was significantly higher among patients with malignant pancreatic tumors than those with inflammatory masses. Normal pancreatic tissue showed a mean strain ratio of 1.68 (95%CI: 1.59-1.78). Inflammatory masses presented a strain ratio (mean 3.28; 95%CI: 2.61-3.96) significantly higher than that of the normal pancreas (P<0.001), but lower than that of pancreatic adenocarcinoma (mean 18.12; 95%CI: 16.03-20.21) (P<0.001). The highest strain ratio was found among endocrine tumors (mean 52.34; 95%CI: 33.96- 70.71). The sensitivity and specificity of the strain ratio for detecting pancreatic malignancies using a cut-off value of 6.04 were 100% and 92.9%, respectively, exceeding the accuracy obtained with qualitative elastography.38 Another recent publication retrospectively evaluated 109 patients with solid pancreatic masses using the same methodology. The final diagnosis was 20 patients with CP (6 without and 7 with focal inflammatory masses, and 7 with autoimmune pancreatitis), 72 with pancreatic cancer, 9 with pancreatic neuroendocrine tumors in 9, and 8 with normal pancreas. In the qualitative evaluation, all pancreatic cancers showed intense blue coloration, whereas the inflammatory masses presented mixed colorations (green, yellow, and low-intensity blue). The mean strain ratio was 23.66 ± 12.65 for the inflammatory masses and 39.08 ± 20.54 for pancreatic cancer (P<0.05).47 The differentiation between mass-forming autoimmune pancreatitis and malignant lesions has been specifically evaluated in one study that comprised 5 patients with mass-forming autoimmune pancreatitis, 17 patients with ductal adenocarcinoma, and 10 healthy subjects.48 The tiff appearance of the mass lesion and the surrounding pancreatic parenchyma distinguishes autoimmune pancreatitis from ductal adenocarcinoma and normal pancreas.
Saftoiu et al.35 investigated quantitative EUS elastography based on hue histograms in their study, which included 22 controls, 11 CP, 32 pancreatic adenocarcinomas, and 3 neuroendocrine tumors. The sensitivity, specificity, positive and negative predictive values, and accuracy of the procedure in differentiating between benign and malignant pancreatic masses were 91.4%, 87.9%, 88.9%, 90.6%, and 89.7%, respectively, using 175 as the cut-off for the mean of the hue histogram. Recently, a multicenter study involving 258 patients (211 with pancreatic adenocarcinoma and 47 with CP) and using the same methodology has been published. The sensitivity, specificity, positive and negative predictive values, and accuracy were 93.4%, 66.0%, 92.5%, 68.9%, and 85.4% respectively, using the same cut-off value (175) for the mean of the hue histogram.49 Schrader et al. investigated quantitative elastography based on the mean of the hue histogram in 86 patients with malignant pancreatic masses and 28 controls without pancreatic diseases.50 A 100% sensitivity and specificity for malignancy detection was obtained through the quantitative measurement of the blue color. However, this study did not include controls with benign pancreatic masses or CP We have compared the two different modalities of quantitative EUS elastography in terms of the strain ratio and hue histogram in a recent study and found no differences in the accuracy for the differentiation between benign and malignant pancreatic masses (unpublished data).
Figure 4 shows the elastographic evaluation of different solid pancreatic masses.
Figure 4.

Quantitative EUS elastography showing different hue histogram (A) and strain ratio (B) values, as well as the basal appearance of pancreatic solid lesions. I) Pancreatic adenocarcinoma; II) Inflammatory mass in chronic pancreatitis; III) Neuroendocrine pancreatic tumor.
Chronic pancreatitis
To date, only one study has been published on elastographic findings among patients with CP.45 In the current study, the qualitative elastography of CP patients demonstrated irregular coloration, showing green areas with heterogenic, predominantly blue strands. These changes were clearly different from those observed in the control group (patients without pancreatic diseases), presented predominantly green and yellow homogeneous patterns. In our experience, normal pancreas presents a homogeneous, predominantly green pattern, and patients with CP present an irregular and heterogeneous, predominantly green pattern, with associated isolated mixed areas (yellow and blue). We have recently conducted a study in our department, evaluating quantitative EUS elastography (based on the calculation of strain ratio) for diagnosing CP. The study comprised 178 patients. The EUS CP criteria were recorded and the patients were classified according to the Rosemont classification. The strain ratios between Rosemont categories revealed significant statistical differences as follows: 1.80 (95%CI: 1.73-1.80) in normal pancreas; 2.40 (95%CI: 2.21-2.56) in cases “indeterminate for CP”; 2.85 (95%CI: 2.69-3.02) for cases suggestive of CP; and 3.62 (95%CI: 3.24-3.99) for cases “consistent with CP” (P<0.001). We also observed a high correlation between the total number of EUS criteria for CP and the strain ratio (r=0.801; P<0.0001)51 (Figure 5).
Figure 5.
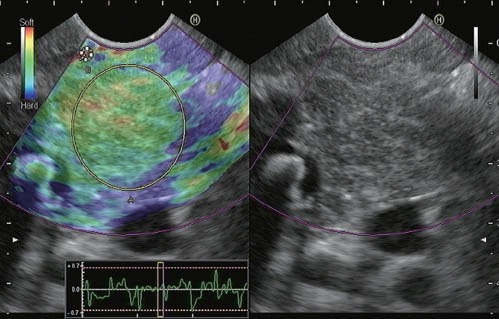
Quantitative EUS elastographic evaluation of a patient with EUS findings suggestive of chronic pancreatitis
Lymph nodes
Giovannini et al.42 analyzed 31 lymph nodes from 25 patients; 3 from the cervical area, 17 from the mediastinum, 5 from the celiac arterial trunk region, and 6 from the aortocaval region. The results of the qualitative EUS elastography were consistent with malignancy in 22 cases, with benign masses in 7 cases, and indeterminate in 2 cases. The indeterminate lesions, which showed heterogenicity, were ultimately classified as benign. No false-negative findings were found, but 5 false-positives were documented. The sensitivity and specificity for determining malignancy were 100% and 50%, respectively. A subsequent multicenter study, also by Giovannini et al., investigated 101 lymph nodes (57 malignant and 44 benign). The elastographic images were interpreted as benign in (score 1+2) cases, indeterminate (score 3) in 10 cases, and malignant (score 4+5) in 53 cases. Considering benign lesions test as negative, and indeterminate and malignant lesions as positive, the sensitivity, specificity, positive predictive value, and negative predictive value for the detection of malignancy were 91.8%, 82.5%, 88.8%, and 86.8%, respectively, whereas the overall accuracy was 88.1%. The interobserver agreement of the evaluation of 30 cases yielded a kappa score of 0.657 for the detection of malignant lymph nodes.43 Jansen et al.52 evaluated the feasibility of qualitative EUS elastography of the dorsal mediastinum, comparing the elastographic patterns of lymph nodes to the gold standard (EUS-guided FNA). A total of 66 lymph nodes were examined (37 benign and 29 malignant under histologic evaluation). In the 31 of 37 benign lymph nodes, elastography showed a homogeneous pattern (intermediate elasticity). Predominantly hard tissues (variable patterns) were found in 23 of the 29 malignant lymph nodes. The three examiners had accuracies ranging from 81.8% to 87.9% for benign lymph nodes and from 84.6% to 86.4% for malignant ones. The interobserver agreement was almost perfect (kappa=0.84). A study on qualitative EUS elastography for evaluating lymph nodes has also been conducted at our institution. A total of 63 lymph nodes (54 mediastinal and 9 abdominal, 31 were malignant and 31 were benign in final diagnosis) from 57 patients were included. Three different elastographic patterns were identified: a predominantly blue pattern, a predominantly green pattern, and a mixed pattern (blue and green without predominance). Of the 31 malignant lymph nodes, 24 showed a predominantly blue pattern and 7 showed a mixed pattern. No malignant lymph nodes were observed with a green pattern. Of the 32 benign lymph nodes, the elastographic patterns of 23 cases were predominantly green, 2 cases were predominantly blue, and in 7 cases were mixed (Figure 6). In other words, the probability of a benign histology in lymph nodes that present a green pattern on elastography is 100%, and the probability of malignant histology with a predominantly blue pattern was 92.3%. In cases presenting a mixed pattern on elastography, the probability of malignant histology was 50%.53 Cervical, mediastinal, or abdominal lymph node EUS elastography has been investigated by Satfoiu et al. in a series of 42 lymph nodes. The sensitivity, specificity, and accuracy for the differentiation between benign and malignant lymph nodes were 91.7%, 94.4%, and 92.86%, respectively.54 The differentiation between benign and malignant lymph nodes has been investigated in one recent meta-analysis that included 7 studies and 368 patients with 431 lymph nodes in total. The pooled sensitivity of EUS elastography for the differential diagnosis of benign and malignant lymph nodes was 88%, and the specificity was 85%. The area under the summary receiver operating characteristic curve was 0.9456. The authors concluded that EUS elastography is a promising, non-invasive method for the differential diagnosis of malignant lymph nodes, and may become a valuable supplemental method to EUS-guided FNA.55
Figure 6.
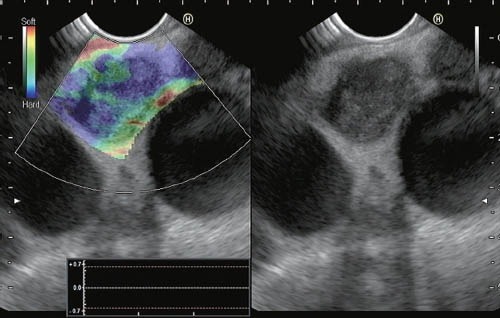
Qualitative EUS elastography of a lymph node patient with EUS findings suggestive of chronic pancreatitis showing a predominantly blue pattern in a patient with non-small cell lung cancer with malignant lymph node metastasis
Studies on quantitative EUS elastography for the evaluation of lymph nodes are few. The previously referred study by Saftoiu et al.54 also included a quantitative analysis based on RGB channel histogram values from EUS elastography images. The sensitivity, specificity, and accuracy for malignancy detection were 95.8%, 94.4%, and 95.2%, respectively, at a cut-off level of 0.84.54 In a subsequent study, Saftoiu et al. investigated 85 cervical, mediastinal, and abdominal lymph nodes in 54 patients using quantitative EUS elastography based on hue histograms. A definitive diagnosis was obtained in 78 cases (37 benign and 41 malignant). The sensitivity, specificity, and accuracy in the detection of malignancy were 85.4%, 91.9%, and 88.5%, respectively, using 166 as the cutoff level for the mean of the hue histogram (between blue and green on the rainbow scale). The corresponding AUC was 0.928.56
Transrectal EUS elastography
The value of transrectal EUS elastography has been investigated for the diagnosis and evaluation of prostate cancer, rectal cancer, inflammatory bowel disease, and fecal incontinence. In prostate cancer, elastography has been demonstrated to be superior to transrectal EUS alone,57 and it improves the specificity of prostate biopsies by highlighting areas highly suspected of malignancy.58 The sensitivity of transrectal elastography in the diagnosis of prostate cancer ranges from 68% to 92%, and its specificity, from 62% to 87%, in patients clinically suspected of prostate cancer.57,58,59,60
Transrectal elastography for differentiating between benign and malignant rectal tumors has been evaluated in one study, which involved 69 patients with rectal tumors. Quantitative elastography using the strain ratio differentiated between adenomas and adenocarcinomas with a sensitivity of 0.93, a specificity of 0.96, and an accuracy of 0.94.61 In a recent pilot study, EUS evaluation of rectal wall thickness and the strain ratio has been investigated for diagnosing inflammatory bowel disease and differentiating Crohn's disease from ulcerative colitis.62 Patients with Crohn's disease had significantly higher strain ratios both than the controls and patients with ulcerative colitis, but there was no difference between the strain ratios of patients with ulcerative colitis and the controls. Allgayer et al. evaluated the elastography of anal sphincters in 50 patients with fecal incontinence, finding no correlation between the elastographic appearance of sphincters and the functional and clinical parameters of the patient.63
Other indications
Given the current indications for conventional EUS, EUS elastography may be useful in evaluating solid lesions in left suprarenal glands by differentiating between adenomas and metastases. Our preliminary unpublished data supports this hypothesis. Another possible indication for EUS elastography is differentiating between benign and malignant solid liver lesions.64,65 Furthermore, the use of EUS elastography for determining the infiltration of adjacent organs in the staging of gastric and esophageal cancers is currently being evaluated in ongoing studies. Further studies will evaluate the usefulness of EUS elastography in diagnosing the aforementioned diseases and other indications in the near future. We believe that EUS elastography will be an integral part of the EUS evaluation of any pathology that may alter tissue stiffness, including inflammation, fibrosis, and cancer.
Conclusion
Qualitative and quantitative EUS elastography are emerging techniques capable of differentiating fibrotic/ inflammatory tissues from malignant lesions. EUS elastography has been demonstrated to differentiate between benign and malignant solid pancreatic masses and lymph nodes with a high accuracy, as well as normal pancreatic tissues from early CP. EUS-guided tissue sampling will still be needed in many situations. However, EUS elastography is useful for identifying cases in which biopsies are unnecessary and for directing biopsies to optimal areas in cases where histologic diagnosis is required. Future research will further define the role of EUS elastography in clinical practice.
Disclosures
Dr. Julio Iglesias-Garcia is an international advisor of Cook-Medical; Dr. J. Enrique Dominguez-Muñoz is an international advisor of Pentax Medical Company.
References
- 1.Dye CE, Waxman I. Endoscopic ultrasound. Gastroenterol Clin North Am. 2002;31:863–79. doi: 10.1016/s0889-8553(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 2.Tamerisa R, Irisawa A, Bhutani MS. Endoscopic ultrasound in the diagnosis, staging, and management of gastrointestinal and adjacent malignancies. Med Clin North Am. 2005;89:139–58. doi: 10.1016/j.mcna.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Byrne MF, Jowell PS. Gastrointestinal imaging: endoscopic ultrasound. Gastroenterology. 2002;122:1631–48. doi: 10.1053/gast.2002.33576. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias García J, Lariño Noia J, Domínguez Muñoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631–8. doi: 10.4321/s1130-01082009000900006. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M. The place of endoscopic ultrasound in bilio-pancreatic pathology. Gastroenterol Clin Biol. 2010;34:436–45. doi: 10.1016/j.gcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Gill KR, Wallace MB. Endoscopic ultrasound and staging of non-small cell lung cancer. Minerva Med. 2007;98:323–30. [PubMed] [Google Scholar]
- 7.De Luca L, Di Bella S, D’Amore E. Mediastinal and gastric EUS: indications and technique examination. Minerva Med. 2007;98:423–9. [PubMed] [Google Scholar]
- 8.Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–79. doi: 10.1016/s0016-5107(04)01529-9. [DOI] [PubMed] [Google Scholar]
- 9.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 10.Turner BG, Cizinger S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS-FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Iglesias-García J, Dominguez-Muñoz JE, Lozano-Leon A, et al. Impact of endoscopic-ultrasound fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289–93. doi: 10.3748/wjg.v13.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 13.Vilmann P, Annema J, Clementsen P. Endosonography in bronchopulmonary disease. Best Pract Res Clin Gastroenterol. 2009;23:711–28. doi: 10.1016/j.bpg.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TQ, Kalade A, Prasad S, et al. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) of mediastinal lesions. ANZ J Surg. 2011;81:75–8. doi: 10.1111/j.1445-2197.2010.05266.x. [DOI] [PubMed] [Google Scholar]
- 15.Erickson RA, Sayage-Rabie L, Beisner RS. Factors’ predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 16.Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: tips for success. Gastrointest Endosc. 2002;56:S86–93. doi: 10.1016/s0016-5107(02)70093-x. [DOI] [PubMed] [Google Scholar]
- 17.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUSguided fine needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 19.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percuteneous FNA. Gastrointest Endosc. 2003;58:690–5. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 20.Eloubeidi MA, Tamhane A, Varadajulu S. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622–9. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Kitano M, Kudo M, Sakamoto H, et al. Endoscopic ultrasonography and contrast-enhanced endoscopic ultrasonography. Pancreatology. 2011;11:28–33. doi: 10.1159/000323493. [DOI] [PubMed] [Google Scholar]
- 22.Saftiou A. State-of-the-art imaging techniques in endoscopic ultrasound. World J Gastroenterol. 2011;17:691–6. doi: 10.3748/wjg.v17.i6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh A, Ueno E, Tohno E, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 24.Cochlin DL, Ganatra RH, Griffiths DFR. Elastography in the detection of prostatic cancer. Clin Radiol. 2002;57:1014–20. doi: 10.1053/crad.2002.0989. [DOI] [PubMed] [Google Scholar]
- 25.Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrasonic Imaging. 1998;20:260–74. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 26.Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Prac Res Clin Gastroenterol. 2009;23:767–79. doi: 10.1016/j.bpg.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhari MH, Forsberg F, Voodarla A, et al. Breast tumor vascularity identified by contrast enhanced ultrasound and pathology: initial results. Ultrasonics. 2000;38:105–9. doi: 10.1016/s0041-624x(99)00146-8. [DOI] [PubMed] [Google Scholar]
- 28.Fornage BD. Recent advances in breast sonography. JBR-BTR. 2000;83:75–80. [PubMed] [Google Scholar]
- 29.Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 30.Gao L, Parker KJ, Lerner RM, et al. Imaging of the elastic properties of tissue – a review. Ultrasound Med Biol. 1996;22:959–97. doi: 10.1016/s0301-5629(96)00120-2. [DOI] [PubMed] [Google Scholar]
- 31.Ophir J, Cespedes EI, Garra BS, et al. Elastography: ultrasound imaging of tissue strain and elastic modulus in vivo. Eur J Ultrasound. 1996;3:49–70. [Google Scholar]
- 32.Giovannini M. Endoscopic Ultrasound Elastography. Pancreatology. 2011;11:34–9. doi: 10.1159/000323496. [DOI] [PubMed] [Google Scholar]
- 33.Frey H. Real-time elastography. A new ultrasound procedure for the reconstruction of tissue elasticity. Radiologie. 2003;43:850–5. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, et al. Endoscopic Ultrasound Elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–8. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Săftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS-elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–94. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira T, Rasband W. The ImageJ User Guide – Version 1.44. Available from http://imagej.nih.gov/ij/docs/user-guide.pdf .
- 37.Hirooka Y, Itoh A, Kawashima H, et al. Diagnosis of pancreatic disorders using contrast-enhanced endoscopic ultrasonography and endoscopic elastography. Clin Gastroenterol Hepatol. 2009;7:S63–7. doi: 10.1016/j.cgh.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 38.Iglesias-Garcia J, Lariño-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: an accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172–80. doi: 10.1053/j.gastro.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 39.Seicean A. Endoscopic Ultrasound in chronic pancreatitis: where are we now? World J Gastroenterol. 2010;16:4253–63. doi: 10.3748/wjg.v16.i34.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brugge WR. Evaluation of pancreatic cystic lesions with EUS. Gastrointest Endosc. 2004;59:698–707. doi: 10.1016/s0016-5107(04)00175-0. [DOI] [PubMed] [Google Scholar]
- 41.Galasso D, Carnuccio A, Larghi A. Pancreatic cancer: diagnosis and endoscopic staging. Eur Rev Med Pharmacol Sci. 2010;14:375–85. [PubMed] [Google Scholar]
- 42.Giovannini M, Hookey LC, Bories E, et al. Endoscopic Ultrasound elastography: the first step towards virtual biopsy? preliminary results in 49 patients. Endoscopy. 2006;38:344–8. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 43.Giovannini M, Botelberge T, Bories E, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;147:1123–6. [PubMed] [Google Scholar]
- 45.Janssen J, Schlörer E, Greiner L. EUS-elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–8. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 46.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–7. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 47.Itokawa F, Itoi T, Sofuni A, et al. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843–53. doi: 10.1007/s00535-011-0399-5. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich CF, Hirche TO, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–20. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 49.Saftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: a multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 50.Schrader H, Wiese M, Ellrichmann M, et al. Diagnostic value of quantitative eus elastography for malignant pancreatic tumors: relationship with pancreatic fibrosis. Ultraschall Med. 2011 doi: 10.1055/s-0031-1273256. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Iglesias-Garcia J, Lariño-Noia J, Dominguez-Muñoz JE. Elastography in the evaluation of chronic pancreatitis. Gastroenterol Hepatol. 2011;34:629–34. doi: 10.1016/j.gastrohep.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952–7. doi: 10.1055/s-2007-966946. [DOI] [PubMed] [Google Scholar]
- 53.Lariño-Noia J, Iglesias-García J, Álvarez-Castro A, et al. Usefulness of endoscopic ultrasound (EUS) elastography for the detection of malignant infiltration of mediastinal and abdominal lymph nodes. Gastroenterology. 2009;5:A–44. [Google Scholar]
- 54.Săftoiu A, Vilmann P, Hassan H, et al. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535–42. doi: 10.1055/s-2006-927117. [DOI] [PubMed] [Google Scholar]
- 55.Xu W, Shi J, Zeng X, et al. EUS elastography for the differentiation of benign and malignant lymph nodes: a meta-analysis. Gastrointest Endosc. 2011;74:1001–9. doi: 10.1016/j.gie.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Săftoiu A, Vilmann P, Ciurea T, et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 57.Kamoi K, Okihara K, Ochiai A, et al. The utility of transrectal real-time elastography in the diagnosis of prostate cancer. Ultrasound Med Biol. 2008;34:1025–32. doi: 10.1016/j.ultrasmedbio.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Kapoor A, Mahajan G, Sidhu BS. Real-time elastography in the detection of prostate cancer in patients with raised psa level. Ultrasound Med Biol. 2011;37:1374–81. doi: 10.1016/j.ultrasmedbio.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Giurgiu CR, Manea C, Crisan N, et al. Real-time sonoelastography in the diagnosis of prostate cancer. Med Ultrason. 2011;13:5–9. [PubMed] [Google Scholar]
- 60.Miyagawa T, Tsutsumi M, Matsumura T, et al. Real-time elastography for the diagnosis of prostate cancer: evaluation of elastographic moving images. Jpn J Clin Oncol. 2009;39:394–8. doi: 10.1093/jjco/hyp026. [DOI] [PubMed] [Google Scholar]
- 61.Waage JE, Havre RF, Odegaard S, et al. Endorectal elastography in the evaluation of rectal tumours. Colorectal Dis. 2011;13:1130–7. doi: 10.1111/j.1463-1318.2010.02440.x. [DOI] [PubMed] [Google Scholar]
- 62.Rustemovic N, Cukovic-Cavka S, Brinar M, et al. A pilot study of transrectal endoscopic ultrasound elastography in inflammatory bowel disease. BMC Gastroenterol. 2011;11:113. doi: 10.1186/1471-230X-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allgayer H, Ignee A, Dietrich CF. Endosonographic elastography of the anal sphincter in patients with fecal incontinence. Scand J Gastroenterol. 2010;45:30–8. doi: 10.3109/00365520903383251. [DOI] [PubMed] [Google Scholar]
- 64.Iglesias-García J, Lariño-Noia J, Souto R, et al. Endoscopic ultrasound (EUS) elastography of the liver. Rev Esp Enferm Dig. 2009;101:717–9. doi: 10.4321/s1130-01082009001000007. [DOI] [PubMed] [Google Scholar]
- 65.Rustemovic N, Hrstic I, Opacic M, et al. EUS elastography in the diagnosis of focal liver lesions. Gastrointest endosc. 2007;66:823–4. doi: 10.1016/j.gie.2007.06.047. [DOI] [PubMed] [Google Scholar]


