Abstract
Accurate diagnosis and subtyping of lymphoma have important prognostic implications and are generally required for treatment planning. Histological assessment, immunophenotyping, and genetic studies are usually necessary. Endoscopic ultrasound guided-fine needle aspiration cytology (EUS-FNAC) is a minimally invasive technique widely used for the evaluation of deep-seated benign and malignant lesions. However, the value of cytological samples in lymphoma diagnosis is still a matter of debate. Endoscopic ultrasound guided-fine needle biopsy (EUS-FNAB) can provide tissue core samples that may help overcome the limitations of cytology. The aim of this review is to summarize the available literature regarding EUS-FNAC and EUS-FNAB for the diagnosis and subtyping of lymphoma. In addition, we discuss its usefulness in the management of primary extra-nodal lymphomas, as well as technical issues that may influence sample quality.
Keywords: endoscopic ultrasound, fine needle aspiration, biopsy, cytology, lymphoma
Introduction
The diagnosis of lymphoma has become increasingly complex due to the rapid expansion of the use of immunological and molecular techniques. The latest classification by the World Health Organization (2008) lists 70 different forms of lymphoma.1 The different types of lymphoma have little in common with each other, with different treatment protocols and variable prognosis. Therefore, a correct diagnosis and classification of the disease is mandatory before initiating treatment. Currently, diagnosis of lymphoma is based upon the evaluation of histological, immunophenotypic, and genetic studies interpreted in the context of the clinical scenario. However, this process is sometimes cumbersome because of the inaccessibility of deepseated lymph nodes or organs (e.g., pancreas) and due to risk of complications with the percutaneous approach.2 Achieving sufficient tissue is crucial. Therefore, invasive and costly procedures, such as thoracotomy, laparotomy, mediastinoscopy, or laparoscopy, may be required.
EUS-guided fine needle aspiration cytology (EUS-FNAC) and biopsy (EUS-FNAB) are excellent techniques for obtaining adequate materials for cytological or histological diagnosis of various lesions.3,4 The advantages of EUS over other imaging techniques include real-time puncture, reduced risk of complications due to the proximity of the needle to the lesion, and the ability to sample small lesions that might be hard to sample using other methods. Finally, EUS allows access to deep-seated lesions, which is a challenge with other techniques. EUS makes sampling of mediastinal, retroperitoneal, and perigastrointestinal lymph nodes possible, with an overall accuracy between 65% and 100%.5 The present manuscript summarizes the available evidence related to the use of EUS-FNAC or EUS-FNAB for the diagnosis of lymphoma, including the classification of lymphoma subtypes according to the most recent classification systems. In addition, we discuss the utility of EUS-FNA in the diagnosis of primary extranodal lymphomas and technical issues related to optimization of tissue acquisition.
FNA for the diagnosis of lymphoma
Several reports have described the efficacy of FNA cytology in the diagnosis of lymphoma. In radiological studies, sensitivity and accuracy range from 66% to 90% and 60% to 80%, respectively.6,7,8,9 Ancillary techniques, such as flow cytometry (FC) applied to conventional cytomorphological analysis (CA), significantly improve diagnostic accuracy.10,11 However, the value of FNA in the diagnosis of lymphoma remains controversial. Some authors claim that CA combined with immunophenotyping by FC can obviate more invasive procedures in the evaluation of this disease.10,11,12 FNAC is particularly useful when combined with FC at differentiating reactive B cells from monoclonal B-cell neoplasms; as a result, many centers used FNA as an initial screening test.13 FC may also be helpful in the immunological subtyping of the lymphoma, although some subtypes cannot be reliably diagnosed; consequently, the evaluation of tissue core biopsies remains the standard criterion for the final diagnosis.1 The limitations of FC include difficulties in the diagnosis of T-cell lymphoma because these cells usually express markers found normally in mature T-cells and in Hodgkin lymphoma, rarity of Reed-Steinberg cells, and absence of monoclonality.14,15
Is it possible to diagnose lymphoma by EUS-FNA?
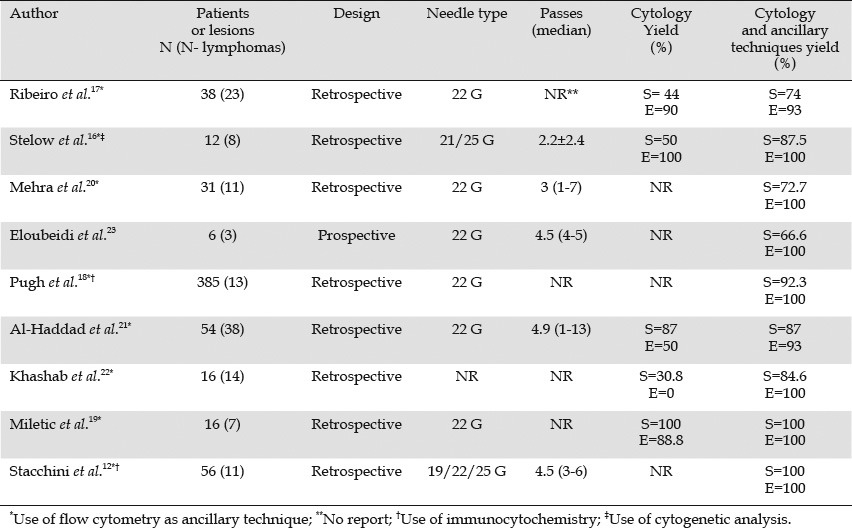
Nine studies have addressed the efficacy of EUS-FNA combined with ancillary techniques in the diagnosis of lymphoma (Table 1).10,11 Of these studies, five included exclusively nodal lymphomas,12,16,17,18,19 two included nodal and extranodal lymphomas20,21 and two included only extranodal lymphomas.22,23 All of them, except one with a small number of patients, had a retrospective design.23 Most studies used the conventional 22 G needle, and the number of passes was different across the studies (Table 1). FC was used in all the studies as an ancillary technique, whereas cytogenetic analysis and immunocytochemistry were performed occasionally. As shown in Table 1, the addition of ancillary techniques, mainly FC, increased the sensitivity considerably (72.7% to 100%), as well as the specificity (93% to 100%) as compared with the cytomorphologic assessment alone (sensitivity and specificity ranging from 30.8% to 87% and from 0% to 100%, respectively). Most of the lymphomas in these series were detected. These data are interesting because they demonstrate that FC is safe (no complications were reported after EUS-FNAC in these studies), very sensitive, and requires only a small amount of tissue (that was obtainable in most cases by EUS-FNAC).12,16,18,20,22 False negative results were attributed to an insufficient amount of material for FC,17,18,22 sampling error,23 or tumor cell destruction owing to the fragility of large cells in large B cell lymphoma.18
Table 1.
Diagnostic yield of EUS-FNA with or without ancillary techniques
Is it possible to subclassify lymphoma by EUS-FNA?
Five studies have investigated the usefulness of EUS-FNA in the classification of lymphoma (Table 2).12,16,18,22,23 Most studies were retrospective and used small size needles. The number of passes varied across the studies. Subclassification of lymphoma was possible in 66.6% to 87.5% of the cases.12,16,18,22,23 However, these studies have some limitations. First, although long-term clinical follow-up was carried out in all the studies, diagnosis and subtyping were not confirmed universally by histology. This procedure is particularly important, as subtyping assessed by EUS-FNA does not always correlate with excisional biopsy results, and it is well-known that lymphomas can respond partially to different chemotherapies although a particular chemotherapy is not the most appropriate treatment for a specific lymphoma subtype.17,24 Therefore, in some studies, the sensitivity could be overestimated. Another limitation is the inability to adequately grade follicular lymphomas, one of the most frequent subtypes after large B-cell lymphomas. This step is a key prognostic factor to guide patient management.25 In these series, 10 cases of follicular lymphoma were diagnosed by cytomorphologic assessment along with ancillary techniques. No information was available about grading.
Table 2.
Efficacy of EUS-FNA for lymphoma classification
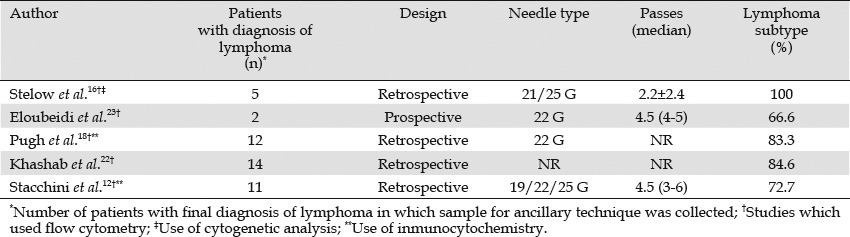
False negative results deserve some comment. In seven cases (16%), ancillary techniques, mainly FC, failed to subtype the lymphoma.12,18,22,23 In three cases, the final diagnosis was large B-cell lymphoma;18,22,23 in one case, Hodgkin lymphoma;12 and in three cases, unspecified B-cell lymphomas.12 In another patient with a final diagnosis of follicular lymphoma, the material was insufficient for FC analysis.22
Therefore, although EUS-FNA along with ancillary techniques (mainly FC) can be a useful tool for lymphoma subtyping, the evidence is still weak because of the small number of reported cases and the lack of a “gold standard.” Consequently, the published data currently suggest that EUS-FNA-based techniques could be of limited value in the diagnosis of Hodgkin lymphoma and in the grading of follicular lymphoma.
How useful are core samples obtained by EUS-FNAB in the diagnosis and subclassification of lymphoma?
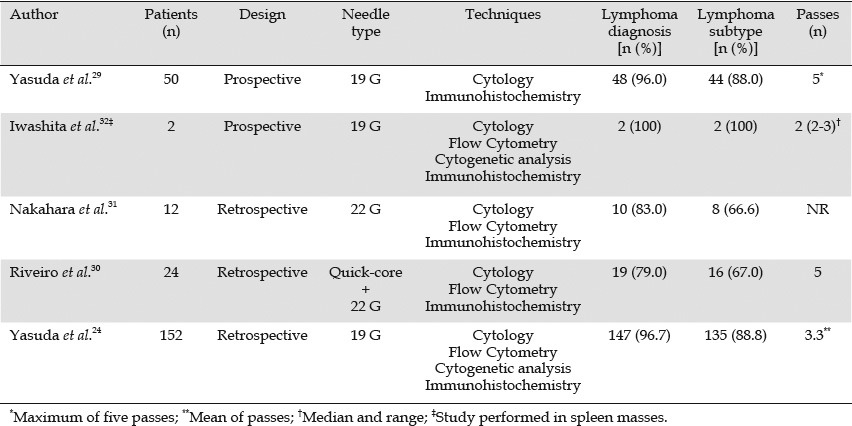
Subclassification of lymphomas is conventionally based on histological findings, and this information is imperative for planning treatment. Excisional biopsy of a node is traditionally performed to provide sufficient tissue for histologic, immunologic, molecular biologic assessment, and classification of lymphomas. As a result, the usefulness of samples obtained by EUS-FNA may be questioned.26,27 However, in patients with nodes in deep sites or masses in retroperitoneal organs, EUS-FNAB can provide core samples.28 Five studies have evaluated the ability of EUS-FNAB to provide adequate core samples for the subclassification of lymphoma(Table 3).24,29,30,31,32 Overall, 240 patients with lymphoma have been reported. The series differ in the design (prospective or retrospective), techniques used (FC, cytogenetic analysis, and histological assessment), needle size (19 G or 22 G) or needle type (trucut biopsy needle vs. regular FNA needle), and number of needle passes. Adequate tissue samples were provided in most cases. Lymphoma diagnosis was achieved in 94% of cases, and subclassification according to the WHO criteria was possible in 85% of cases. Less than six passes were required for lymphoma diagnosis and subclassification in most cases.
Table 3.
Studies using EUS-guided core sample (EUS-FNB) for lymphoma diagnosis and subtyping
In 29 patients, subclassification was not possible. Most cases were large B-cell lymphomas (n=7), follicular lymphomas (n=6), Hodgkin lymphoma (n=4), and mantle cell lymphoma (n=3). However, despite large B-cell lymphoma being the most common subtype found in the series (n=98, 41%), it accounted for less than 25% of the lymphomas subclassified inadequately by EUS-FNAB. These data are in accordance with the study of Ribeiro et al.,30 where a higher yield of EUS-FNAB was found in large B-cell lymphomas than non-large B-cell lymphomas (79% vs. 67%). Interestingly, Yasuda et al. reported a success rate of 85.7% (36 of 42 cases) in the grading of follicular center cell lymphoma.24
False negative results for lymphoma diagnosis were attributed to massive tumor necrosis in some cases,30 insufficient material,31 or technical limitations related to the use of large needles, and needles (trucut needle malfunction) that led to limited tissue penetration.30
Overall, seven complications were reported (2.9%): three cases of submucosal hematoma, one case of mild abdominal pain, two cases of fever, and one case of variceal bleeding.24,30,31,32 Conservative management was sufficient for the first six cases, but the last case had a fatal outcome (presumably not related to the procedure).31 Based on these data, EUS-FNAB may be a useful technique for diagnosis and subtyping of lymphoma.
Primary lymphomas in deep-seated organs
Some EUS-FNA series have included primary pancreatic or splenic lymphoma. Primary pancreatic lymphoma (PPL) is a rare disease that represents approximately 1% of extranodal lymphomas and 0.5% of all pancreatic masses. When suspected, it is crucial to achieve the correct diagnosis, because management and prognosis of PPL are completely different from pancreatic adenocarcinoma. Only one published series addressed the feasibility of EUS-FNA and FC in PPL (Tables 2 and 3). The diagnosis and classification of lymphoma were possible in most cases.
For splenic tumors, lymphoma is the most common diagnosis.2 In a large series using percutaneous biopsy, the complication rate was 5.3%, including pneumothorax, hemoperitoneum, and subacute bleeding. EUS-FNAC or FNAB can overcome this limitation. Although the experience with EUS-FNA and ancillary techniques in this setting is very limited (Tables 1 and 3),23,32 it suggests that diagnosis and subtyping of spleen lymphoma are possible, with low risk of complications.
Technical EUS issues
Needle size
It remains unclear whether the use of large-sized needles (i.e., 19 G needles) increases the diagnostic yield for lymphoma. However, to obtain an adequate core sample for histological evaluation (preserving tissue architecture), large FNA needles (e.g., conventional 19 G FNA needle, trucut needle [Quick core ™], 19 G ProCore™ needle) seem to be better than smaller gauge needles.33 Although the trucut needle has been useful in the diagnosis of lesions in which histological assessment may be important as in gastrointestinal stromal tumors or lymphomas,34,35 it does not perform well when the echoendoscope is not straight (e.g., duodenal bulb approach). Regarding the conventional 19 G FNA needle, several studies have shown its ability to provide histological specimens sufficient to diagnose sarcoidosis or lymphoma and to perform ancillary techniques.24,29,32,36 Scarce, non-comparative data suggest that a new 19 G ProCore™ needle can provide core biopsies and can overcome the drawbacks of the trucut needle. A recent multicenter study in 109 consecutive patients with different types of solid lesions reported an adequacy for histological assessment of 89% and a diagnostic accuracy of 86% with the 19 G ProCore™ needle.37
In the studies carried out in lymphoma (Table 3), the conventional 19 G needle was used in most cases without failure.24,29,32 However, the trucut needle failed in two patients with periduodenal lesions.30
Other EUS-FNA issues
The number of passes required for diagnosis has not been well established. However, the number of passes ranges from two to five in all published studies.24,29,30,31,32
Although many media are available for FC Hank's balance, salt solution or Roswell Park Memorial Institute medium are most frequently used.
Finally, the necessity for on-site cytology is questionable. The presence of the cytologist is helpful to exclude epithelial cancer and to confirm the need to take samples for tests required to diagnose lymphoma (e.g., FC and core biopsy, among others). However, the presence of the cytologist may not improve the yield of EUS-FNA for lymphoma, because obtaining adequate lymphoid tissue from lymph nodes is usually not difficult (as long as enough passes are performed). When the cytologist sees only lymphoid tissue during on-site analysis, it is impossible to diagnose most lymphomas until FC is performed. Therefore, when lymphoma is suspected and an epithelial cancer is very unlikely (e.g., large nodes but no obvious epithelial primary tumor), it may be reasonable to forgo on-site cytological analysis and to perform four or five passes for FC and for regular cytology. For histological samples, onsite analysis is not possible, so the issue is moot.
Conclusion
Accurate lymphoma diagnosis and subtyping are possible by EUS-FNAC and/or EUS-FNAB. These techniques are a reasonable first choice when superficial nodes or lesions are not accessible.
Disclosures
The authors declared no conflicts of interest.
Acknowledgement
This work was supported by a grant from the Fundación Alfonso Martín Escudero (convocatoria 2010) and grant from the Egyptian government “Postdoctoral scientific mission 2010”. Funding sources had no involvement in study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the paper for publication.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. 4th edn. Lyon: IARC Press; 2008. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. [Google Scholar]
- 2.Civardi G, Vallisa D, Berte R, et al. Ultrasound-guided fine needle biopsy of the spleen: high clinical efficacy and low risk in a multicenter Italian study. Am J Hematol. 2001;67:93–9. doi: 10.1002/ajh.1085. [DOI] [PubMed] [Google Scholar]
- 3.Crowe DR, Eloubeidi MA, Chhieng DC, et al. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180–5. doi: 10.1002/cncr.21912. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357–61. doi: 10.1053/ge.1999.v50.97208. [DOI] [PubMed] [Google Scholar]
- 5.Puli SR, Batapati Krishna Reddy J, et al. Endoscopic ultrasound: it's accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cafferty LL, Katz RL, Ordonez NG, et al. Fine needle aspiration diagnosis of intraabdominal and retroperitoneal lymphomas by a morphologic and immunocytochemical approach. Cancer. 1990;65:72–7. doi: 10.1002/1097-0142(19900101)65:1<72::aid-cncr2820650116>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco CH, Richli WR, Lawrence D, et al. Fine needle aspiration biopsy in lymphoma. Radiol Clin North Am. 1990;28:879–83. [PubMed] [Google Scholar]
- 8.Cartagena N, Jr, Katz RL, Hirsch-Ginsberg C, et al. Accuracy of diagnosis of malignant lymphoma by combining fine-needle aspiration cytomorphology with immunocytochemistry and in selected cases, Southern blotting of aspirated cells: a tissue-controlled study of 86 patients. Diagn Cytopathol. 1992;8:456–64. doi: 10.1002/dc.2840080506. [DOI] [PubMed] [Google Scholar]
- 9.Das DK. Value and limitations of fine-needle aspiration cytology in diagnosis and classification of lymphomas: A review. Diagn Cytopathol. 1999;21:240–9. doi: 10.1002/(sici)1097-0339(199910)21:4<240::aid-dc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Meda BA, Buss DH, Woodruff RD, et al. Diagnosis and subclassification of primary and recurrent lymphoma. The usefulness and limitations of combined fine-needle aspiration cytomorphology and flow cytometry. Am J Clin Pathol. 2000;113:688–99. doi: 10.1309/0Q7F-QTGM-6DPD-TLGY. [DOI] [PubMed] [Google Scholar]
- 11.Young NA, Al-Saleem TI, Ehya H, et al. Utilization of fine-needle aspiration cytology and flow cytometry in the diagnosis and subclassification of primary and recurrent lymphoma. Cancer. 1998;84:252–61. [PubMed] [Google Scholar]
- 12.Stacchini A, Carucci P, Pacchioni D, et al. Diagnosis of deep-seated lymphomas by endoscopic ultrasound-guided fine needle aspiration combined with flow cytometry. Cytopathology. 2012;23:50–6. doi: 10.1111/j.1365-2303.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinfort DP, Conron M, Tsui A, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol. 2010;5:804–9. doi: 10.1097/jto.0b013e3181d873be. [DOI] [PubMed] [Google Scholar]
- 14.Al Shanqeety O, Mourad WA. Diagnosis of peripheral T-cell lymphoma by fine-needle aspiration biopsy: a cytomorphologic and immunophenotypic approach. Diagn Cytopathol. 2000;23:375–9. doi: 10.1002/1097-0339(200012)23:6<375::aid-dc2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Young NA, Al-Saleem T. Diagnosis of lymphoma by fine-needle aspiration cytology using the revised European-American classification of lymphoid neoplasms. Cancer. 1999;87:325–45. doi: 10.1002/(sici)1097-0142(19991225)87:6<325::aid-cncr3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Stelow EB, Lai R, Bardales RH, et al. Endoscopic ultrasound-guided fine-needle aspiration of lymph nodes: the Hennepin County Medical Center experience. Diagn Cytopathol. 2004;30:301–6. doi: 10.1002/dc.10405. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 18.Pugh JL, Jhala NC, Eloubeidi MA, et al. Diagnosis of deep-seated lymphoma and leukemia by endoscopic ultrasound-guided fine-needle aspiration biopsy. Am J Clin Pathol. 2006;125:703–9. doi: 10.1309/9C8B-78K0-X27U-77DC. [DOI] [PubMed] [Google Scholar]
- 19.Miletic Z, Gizdic B, Stoos-Veic T, et al. Flow cytometric analysis of deep-seated lymph nodes. Coll Antropol. 2010;34:377–80. [PubMed] [Google Scholar]
- 20.Mehra M, Tamhane A, Eloubeidi MA. EUS-guided FNA combined with flow cytometry in the diagnoses of suspected or recurrent intrathoracic or retroperitoneal lymphoma. Gastrointest Endosc. 2005;62:508–13. doi: 10.1016/j.gie.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Al-Haddad M, Savabi MS, Sherman S, et al. Role of endoscopic ultrasound-guided fine-needle aspiration with flow cytometry to diagnose lymphoma: a single center experience. J Gastroenterol Hepatol. 2009;24:1826–33. doi: 10.1111/j.1440-1746.2009.06005.x. [DOI] [PubMed] [Google Scholar]
- 22.Khashab M, Mokadem M, DeWitt J, et al. Endoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma - a case series. Endoscopy. 2010;42:228–31. doi: 10.1055/s-0029-1243859. [DOI] [PubMed] [Google Scholar]
- 23.Eloubeidi MA, Varadarajulu S, Eltoum I, et al. Transgastric endoscopic ultrasound-guided fine-needle aspiration biopsy and flow cytometry of suspected lymphoma of the spleen. Endoscopy. 2006;38:617–20. doi: 10.1055/s-2005-921111. [DOI] [PubMed] [Google Scholar]
- 24.Yasuda I, Goto N, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for diagnosis of lymph-oproliferative disorders: feasibility of immunohistological, flow cytometric, and cytogenetic assessments. Am J Gastroenterol. 2012;107:397–404. doi: 10.1038/ajg.2011.350. [DOI] [PubMed] [Google Scholar]
- 25.Hehn ST, Grogan TM, Miller TP. Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046–52. doi: 10.1200/JCO.2004.02.104. [DOI] [PubMed] [Google Scholar]
- 26.Armitage JO. How I treat patients with diffuse large B-cell lymphoma. Blood. 2007;110:29–36. doi: 10.1182/blood-2007-01-041871. [DOI] [PubMed] [Google Scholar]
- 27.Kwan V, Gottlieb D. Endoscopic ultrasound-fine needle aspiration for the diagnosis of lymphoma: are we there yet? J Gastroenterol Hepatol. 2009;24:1808–9. doi: 10.1111/j.1440-1746.2009.06137.x. [DOI] [PubMed] [Google Scholar]
- 28.Thomas T, Kaye PV, Ragunath K, et al. Endoscopic-ultrasound-guided mural trucut biopsy in the investigation of unexplained thickening of esophagogastric wall. Endoscopy. 2009;41:335–9. doi: 10.1055/s-0029-1214470. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 30.Ribeiro A, Pereira D, Escalon MP, et al. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc. 2010;71:851–5. doi: 10.1016/j.gie.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Nakahara O, Yamao K, Bhatia V, et al. Usefulness of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for undiagnosed intra-abdominal lymphadenopathy. J Gastroenterol. 2009;44:562–7. doi: 10.1007/s00535-009-0048-4. [DOI] [PubMed] [Google Scholar]
- 32.Iwashita T, Yasuda I, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for splenic tumor: a case series. Endoscopy. 2009;41:179–82. doi: 10.1055/s-0028-1119474. [DOI] [PubMed] [Google Scholar]
- 33.Song TJ, Kim JH, Lee SS, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–45. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 34.Storch I, Jorda M, Thurer R, et al. Advantage of EUS Trucut biopsy combined with fine-needle aspiration without immediate on-site cytopathologic examination. Gastrointest Endosc. 2006;64:505–11. doi: 10.1016/j.gie.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 35.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–6. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 36.Iwashita T, Yasuda I, Doi S, et al. The yield of endoscopic ultrasound-guided fine needle aspiration for histological diagnosis in patients suspected of stage I sarcoidosis. Endoscopy. 2008;40:400–5. doi: 10.1055/s-2007-995593. [DOI] [PubMed] [Google Scholar]
- 37.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]


