Abstract
Objective:
Although endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) is the gold standard for diagnosing pancreatic lesions, its negative predictive value is suboptimal. Our aim was to evaluate the yield of contrast-enhanced EUS (CED-EUS) and of strain ratio EUS-elastography (SR-E-EUS) for differentiating pancreatic solid lesions.
Methods:
Forty-seven patients (27 men, 20 women, 70 ± 11 years) were consecutively involved in this single-center, prospective study. They were submitted to EUS, SR-E-EUS, CED-EUS with Sonovue®, and EUS-FNA. The final diagnosis was based on the histological assessment of EUS-FNA and/or surgical specimens when available, and on follow-up of at least 6 months.
Results:
From the 47 focal pancreatic lesions included, 13 (28%) were benign and 34 (72%) malignant. Patients with malignancy were older (70 ± 11 vs. 61 ± 8, P = 0.003), and had larger lesions (34 ± 12 mm vs. 22 ± 11 mm, P = 0.03). Malignant lesions had higher SR-E-EUS (31 ± 32 vs. 8 ± 9, P = 0.001) and more hypovascular pattern (93% vs. 33%, P < 0.001). Logistic regression determined that only hypovascularity (OR = 2.6, 95%CI: 1.5-130, P = 0.02) was independently predictive of malignancy. ROC analysis for SR-E-EUS yielded an optimal cutoff of 8 (AUC 0.91, 95%CI: 0.74-0.98) for the best power distinction for malignancy. There was no significant difference concerning sensitivity (79%, 90%, 93%) and specificity rates (85%, 75%, 67%) of EUS-FNA, SR-E-EUS, and CED-EUS, respectively. By analysis of the inconclusive EUS-FNA subset (9 patients, 19%), SR-E-EUS > 8 and hypovascularity showed sensitivity of 80% and 100%, and specificity of 67% and 67%, respectively.
Conclusion:
The clinical utility of CED-EUS and SR-E-EUS remains questionable. The accuracies of CED-EUS and SR-E-EUS are similar to EUS-FNA. Hypovascularity was independently predictive of malignancy. Patients with inconclusive EUS-FNA could benefit from CED-EUS due to the high sensitivity of hypovascularity for diagnosing malignancy.
Keywords: endosonography, pancreatic neoplasms, elastography, contrast enhanced ultrasound, fine needle aspiration, endoscopic ultrasound, fine needle aspiration
INTRODUCTION
The diagnosis of focal pancreatic lesions is often difficult. Acute or chronic pancreatitis can be indistinguishable from a neoplastic mass based only on imaging. On the other hand, pancreatic cancer usually has an area of adjacent obstructive pancreatitis. Endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) can help this differential diagnosis.
However, false-negative results are obtained in up to 44% with considerable implications.1,2 As a means of trying to overcome this limitation, two techniques have been added to EUS: contrast-enhanced power Doppler EUS (CED-EUS) and elastography (E-EUS).
CED-EUS uses contrast media injection in combination with power Doppler to establish the micro-vascularization pattern of the lesions. It is known that pancreatic adenocarcinoma exhibits reduced contrast enhancement compared with surrounding pancreatic tissue. Initial studies showed that most pancreatic adenocarcinoma were hypoperfused lesions.3,4 Hocke et al. (2006)5 reported that the sensitivity and specificity for the discrimination between benign and malignant pancreatic lesions increased from 73% to 91% and from 83% to 93%, respectively, using second-generation contrast agent (SonoVue®, Bracco Imaging, Milan, Italy). More recently, Saftoiu et al. (2010)6 reported sensitivity and specificity rates of 79% and 88% for CED-EUS.
E-EUS is a real-time imaging procedure used for visualization of tissue elasticity. It is carried out with conventional EUS probes and does not require additional instruments. Inflammatory conditions and tumors lead to an alteration of the normal tissue structure causing hardening. The differences of tissue elastic properties are transformed in a colorized scale. The colors associated with hard, intermediate, and soft tissues are blue, green/yellow, and red, respectively. Initial studies have reported sensitivity from 80% to 100% for the differential diagnosis in pancreatic masses.7,8 Malignant lesions usually appear with a blue/green honeycomb pattern in E-EUS.7,8
The analysis of static images obtained by E-EUS has been questioned due to the subjective interpretation of the elastographic pattern. A quantitative and more objective assessment is provided by the measurement of the strain ratio (SR) between the percentage strain of the target lesion and the percentage strain of the surrounding tissue. Fumihide et al. (2008)9 presented data, in an abstract form, showing a significant difference of the mean SR between chronic pancreatitis and pancreatic cancer. Iglesias-Garcia et al. (2010)10 reported a sensitivity and specificity of SR-E-EUS for detecting malignancy of 100% and 93%, respectively. Recently, the European EUS Elastography Multicentric Study Group proposed that the use of artificial intelligence methodology via artificial neural networks processing the elastography digitalized movies could improve the diagnosis of pancreatic lesions.11
Our aim was to evaluate the yield of CED-EUS using SonoVue® and of SR-E-EUS in differentiating benign and malignant focal pancreatic solid lesions in a clinical setting.
METHODS
Patients
The study included all consecutive patients with a focal pancreatic lesion who underwent an EUS examination at the Institute Paoli-Calmettes, Marseille, France, between June 2008 and November 2008. Patients with cystic masses or neuroendocrine tumors were excluded from the study. Heart failure NYHA III/IV or severe lung disease were exclusion criteria for using contrast agent. There are no contraindications to perform elastography. The study protocol was conducted in accordance with the ethical principles, the guidance of the Helsinki Declaration, and the Good Clinical Practice Guidelines. The study was approved by the local Ethics Committee.
Protocol
EUS procedures were performed by four experienced endosonographers in a typical clinical setting with previous knowledge of the patient's underlying disease. Linear-array echoendoscopes (EG38UT, Pentax Europe Ltd., Hamburg, Germany), an ultrasound platform (Hitachi 7500 or 8500, Hitachi Medical Systems GmbH, Wiesbaden, Germany) with an integrated elastography module were used. EUS, SR-E-EUS, and CED-EUS were performed sequentially during the same EUS examination. The E-EUS and CED-EUS images were read during the examination, with the conclusions being recorded at the end of the examination, before pathological assessment.
Calculation of the tissue elasticity distribution was carried out in real-time fashion. The suitability of the elastographic signal was indicated by a numeric scale from 1 to 7 within the image. Maximal sensitivity for elastographic registration was consistently used in the study. Adequate signals were represented for a scale number equal to or greater than 3. Elastographic and B-mode images were displayed simultaneously side by side. The sample area was adjusted to the region of interest; the maximal depth of the area was about 3.5 cm. To obtain the strain ratio, a circular area was adjusted to the focal lesion and a second one was adjusted to the surrounding tissue. The SR (focal lesion area strain % / surrounding tissue area strain %) was calculated in order to evaluate the objective hardness as a numerical value (Fig. 1, 2). The mean of three measurements was used.
Figure 1.
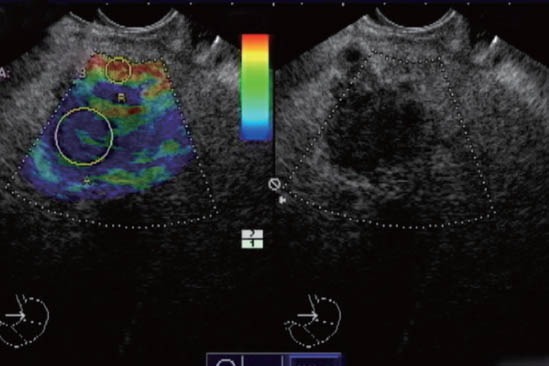
Elastographic and B-mode images are displayed simultaneously side by side. A circular area was adjusted to the focal lesion and a second one was adjusted to the surrounding tissue. The strain ratio of 42.6 was calculated in this case of pancreatic cancer.
Figure 2.
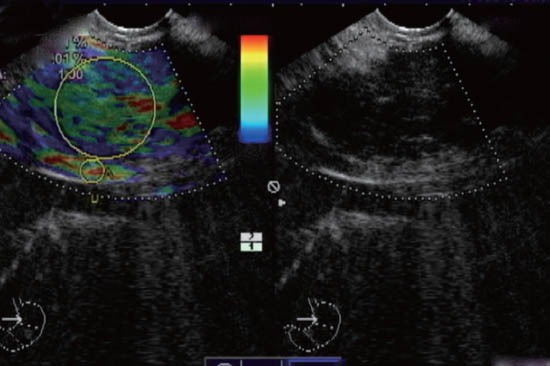
A strain ratio of 1 was calculated in this case of chronic pancreatitis.
After conventional EUS and E-EUS, SonoVue® 2,4 mL (Bracco Imaging, Milan, Italy) was injected intravenously via a catheter (1.2 mm in diameter or larger) into a cubital vein, via the 3-way stopcock, at a rate of 1 mL/s, following a flash of 10 mL saline solution. The enhancement pattern was defined using power Doppler mode by observing for over 3 min (Fig. 3, 4). The criterion for hypovascular pattern was the paucity or absence of vessels by using power Doppler compared to the surrounding tissue.
Figure 3.
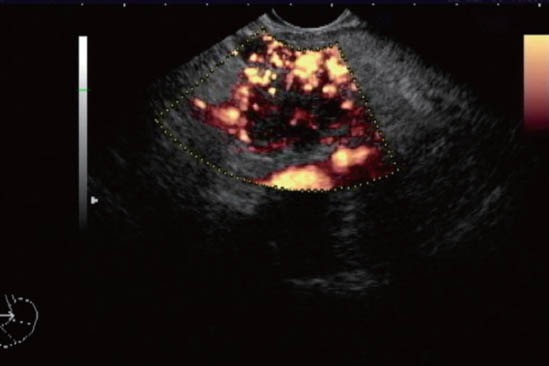
Hypovascular pattern in pancreatic cancer mass examined with power Doppler after contrast injection. The pattern of enhancement pattern was defined by observing for over 3 min.
Figure 4.
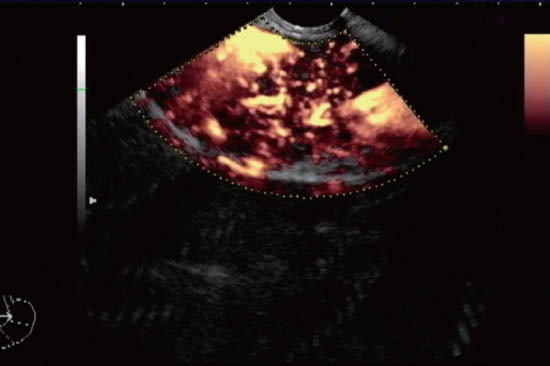
Hypervascular pattern in chronic pancreatitis examined with power Doppler after contrast injection.
EUS-FNA was performed by using a 22-G FNA needle (Echotip, Cook Endoscopy, Winstow-Salem, North Carolina, USA). An immediate screening at the time of EUS-FNA was not performed. Direct smears were prepared by the endoscopist and were stained by May-Grunwald-Giemsa on air dried slides. ThinPrep® preparation (monolayer cytology, Cytyc Corp., Boston, Massachussets, USA) was used in all cases. Cell block material, fixed in 10% neutral buffered formalin, was collected at the reception of the aspirated material. Haematoxylin-eosin staining was performed on cell block preparation and on monolayer cytology slide. Immunohistochemical analysis was performed when necessary.
The final diagnosis was based on the histological assessment of the EUS-FNA samples and/or surgical specimens when available. A positive cytological diagnosis was taken as a final proof of malignancy. For negative cytological specimens, the diagnosis was confirmed by surgery or follow-up by imaging (EUS or computed tomography or magnetic resonance imaging) of at least six months. If the patient was still alive 6 months after the EUS with no signs of disease progression, he or she was considered to have benign disease.
Statistical Analysis
The statistical analysis was done using SPSS 13.0 (SPSS Inc., Chicago) software. The categorical variables were expressed by their absolute (n) and relative frequency (%) and compared using the Chi-squared test or Fisher Exact test. The continuous variables were expressed by mean and standard deviation and compared by using Student's t-test or Mann-Whitney U test. An association was considered to be statistically significant at P < 0.05. Stepwise logistic regression analysis was carried out to search for independent predictors of malignancy. The sensitivity, specificity, positive (PPV) and negative predictive values (NPV), with 95% confidence intervals (95% CI), and overall accuracy were calculated.
Data were analyzed by sensitivity and specificity derived from the receiver operating characteristic (ROC) curve and area under the ROC curve (AUC). The McNemar test was used to compare these calculated sensitivities and specificities.
RESULTS
Fifty patients (27 men, 23 women, mean age 70 ± 11 years) with a focal pancreatic lesion were evaluated. Three patients with neuroendocrine tumors were excluded from the study. From the 47 focal pancreatic lesions included, 13 (28%) were benign and 34 (72%) malignant, with the final diagnosis based on a combination of EUS-FNA results (39 lesions), surgery with pathology results (11 lesions) and follow-up for at least 6 months (13 lesions). From the 13 considered benign lesions, 4 had pathological surgical confirmation.
Final benign diagnoses were chronic pancreatitis (n = 4), auto-immune pancreatitis (n = 1) and non-specific diseases (n = 8). Patients with benign lesions had a mean follow-up of 9 ± 3 months (range: 6-14 months). Final malignant diagnosis were pancreatic adenocarcinoma (n = 33) and pseudopapilar solid tumor (n = 1).
The mean size of the lesions was 31 ± 13 mm (range 7-60 mm). Twenty-four (51%) focal pancreatic lesions were located in the head/uncinate, 13 (28%) in the body, and 10 (21%) in the tail of pancreas. Seven (15%) patients had inadequate elastographic signals and 7 (15%) had contraindications for using contrast agent. No adverse effects were caused by the contrast media.
Comparative analysis between benign and malignant lesions is shown in Table 1. Patients with malignant lesions were older. Malignant lesions were larger, and had greater strain ratio and more hypovascular pattern. The mean strain ratio was 8 ± 9 for benign and 31 ± 32 for malignant lesions (P = 0.001). Hypovascular pattern after contrast injection was present in 93% of malignant and 33% of benign lesions (P < 0.001). Logistic regression analysis determined that only hypovascular pattern [odds ratio (OR) = 2.6, (95% CI: 1.5-130, P = 0.02) was independently predictive of malignancy.
Table 1.
Comparative analysis between benign and malignant pancreatic lesions

ROC analysis for the mean SR-E-EUS of the region of interest yielded an optimal cutoff of 8 with an AUC of 0.91 (95% CI: 0.74-0.98) for the best power distinction for malignancy. It provided a sensitivity and specificity of 90% and 75%, respectively (Fig. 5).
Figure 5.

The receiver operating characteristic (ROC) curve of the mean strain ratio obtained by endoscopic ultrasonography-elastography of the region of interest used for the discrimination between benign and malignant lesions (sensitivity of 90% and specificity of 75% for a cutoff value of 8). The area under the ROC curve was 0.91.
According to the EUS-FNA results, from the 34 malignant lesions, 26 had a positive EUS-FNA, 1 a negative EUS-FNA, and 7 inconclusive results. From the 13 benign lesions, 11 had a negative EUS-FNA and 2 inconclusive results. Therefore, 9 patients (19%) had non-diagnostic EUS-FNA. Considering these results as false (either positive or negative), our sensitivity and specificity rates for EUS-FNA were 79% and 85%, respectively.
The sensitivity, specificity, PPV, NPV, and overall accuracy of all criteria for diagnosing malignancy are shown in Table 2. The sensitivity of SR-E-EUS was not significantly different from CED-EUS (90% vs. 93%, P = 0.50) and both were not significantly different from EUS-FNA (79%, P = 0.93). The specificity of SR-E-EUS was not significantly different from CED-EUS (75% vs. 67%, P = 0.50) and both were not significantly different from EUS-FNA (85% vs. 67%, P = 0.82; and vs. 75%, P = 0.50, respectively).
Table 2.
Performance of the criteria used for differential diagnosis between benign and malignant pancreatic focal lesions
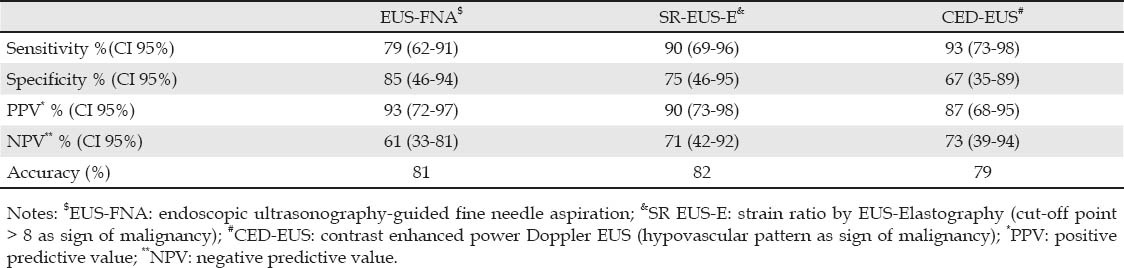
We also analyzed the subset of 9 patients with inconclusive EUS-FNA. From these, 3 (33%) were benign and 6 (67%) were malignant. The performance of SR-EUS-E > 8 criterion (optimal cutoff) as sign of malignancy showed sensitivity, specificity, PPV, NPV, and accuracy rates of 80% (95% CI: 30%-99%), 67% (95% CI: 13%-98%), 80% (95% CI: 23%-99%), 67% (95% CI: 13%-98%), and 74%, respectively. The performance of hypovascular pattern criterion as sign of malignancy showed sensitivity, specificity, PPV, NPV, and accuracy rates of 100% (95% CI: 52%-100%), 67% (95% CI: 13%-98%), 86% (95% CI: 42%-99%), 100% (95% CI: 20%-100%), and 88%, respectively.
DISCUSSION
The diagnosis of focal solid lesions is one of the most difficult problems for those who take care of patients with pancreatic diseases. It has been hypothesized that CED-EUS and EUS-E could help in differentiating benign from malignant lesions. In this study we reported our experience using both techniques combined in a clinical setting. Although the overall accuracy of both techniques was similar and added no gain to EUS-FNA, the subset of patients with inconclusive EUS-FNA could benefit from these new diagnostic modalities. The presence of hypovascular pattern in CED-EUS was independently predictive of malignancy in the logistic regression and had a high sensitivity and NPV in patients with inconclusive EUS-FNA.
For a better sample adequacy neuroendocrine lesions were withdrawn from the study. They have a typical appearance in EUS-E and CED-EUS as previously shown by Dietrich et al. (2008) and by our own group (2009).12,13 They have a hypoechoic region in the center with a green appearance in the small area surrounded by blue or harder tissue and a SR usually less than 10 in EUS-E. They also have a strong contrast enhancement pattern, indicating hypervascular pattern, in CED-EUS. Therefore there is no difficulty in separating them from pancreatitis or adenocarcinoma.
Similar to the inconclusive results for EUS-FNA, both techniques have inherent limitations. Seven patients (15%) had contraindications for using the contrast agent of CED-EUS such as severe heart failure and lung disease. CED-EUS is also limited by multiple tissue artifacts, such as blooming and overpainting with Doppler. The use of contrast-enhanced harmonic imaging techniques can be an option to CED-EUS. Although these techniques are more sensitive, they are still unavailable for EUS because of the limited frequency bandwidth and acoustic power output of current echoendoscopes. Recently, Kitano et al. (2008)14 and Napoleon et al. (2010)15 used prototype echoendoscopes specific for contrast-enhanced harmonic EUS. They successfully visualized microcirculation and vascular structures of the pancreas. Additional studies will be required to test its utility in diagnosing pancreatic diseases and its superiority to the conventional power Doppler imaging techniques.
The first issue to be discussed about EUS-E is the quality of elastographic signals. While Iglesias-Garcia et al. (2010)10 reported that elastography was feasible in all patients; good elastographic signals could not be obtained in 15% in this study. Other authors have also reported some difficulties in obtaining adequate elastographic signals.8 The depth of the elastographic registration is limited in the same way as in B-mode EUS. If the resolution quality of the B-mode image is too low, subsequent calculation of the elastographic image will be impossible. Necrosis within pancreatic lesion may also soften the consistency and consequently result in a red (soft) appearance instead of the usual blue (hard) one. However, the main limitation of the EUS-E seems to arise more from the overlap of similar mechanical properties in normal tissue and tissue affected by benign or malignant disease than from any technical shortcomings of elastography.16
In this study, from 28 malignant lesions that had CED-EUS, 26 (93%) showed a hypovascular pattern. Our high sensitivity for CED-EUS is similar to that demonstrated by Hocke et al. (2008)17 and Dietrich et al. (2008)12. Conversely to us, however, those authors obtained a high specificity for hypovascularity as a sign of malignancy (95%-100%). That discrepancy could be explained by main factors: different endosonographic criteria for defining vascularization pattern, evaluation time, and sample composition. While Hocke et al. (2008)17 stored all images on videotapes that were re-evaluated later, we analyzed the vascularization pattern according to Becker et al. (2001)4 in a real-time fashion. This should have produced a worse effectiveness compared to he efficacy demonstrated by Hocke et al. (2008)17. While we included one third of benign disorders in our sample, Dietrich et al. (2008)12 did not include benign processes which can have contributed for their high specificity.
Studies have shown ambivalent results for EUS-E. Taken as a whole, our sensitivity, specificity, PPV, NPV, and accuracy rates were comparable to those of Janssen et al. (2007).8 In their study, almost all malignant lesions had the same elastographic appearance, i.e., a blue/green honeycomb pattern. Their rates for this pattern indicating malignancy were 94%, 65%, 52%, 97%, and 74%, respectively. On the other side, Hirche et al. (2008)16 predicted the nature of pancreatic lesions with EUS-E with a poor diagnostic sensitivity (41%), specificity (53%), and accuracy (45%). Major reasons for that poor performance were the EUS-E limitations described such as incomplete delineation of border of lesions greater than 35 mm or of lesions at some distance form the transducer. These authors also questioned its clinical use for differential diagnosis since strain images from all kinds of pancreatic masses were found to be harder than the surrounding tissue, irrespective of the underlying nature of the lesion (i.e., benign vs. malignant).
We join those who agree that a simplistic correspondence between color-coded information and histological diagnosis (green equivalent to “benign” and blue equivalent to “malignant”) seems rather difficult to justify.18,19 Although the approach of using SR-EUS-E seems to be more objective and would certainly avoid the perception artifacts induced by the merging of blue into green, it brought no significant gain in this study.
Similar to Iglesias-Garcia et al. (2010)10 and Dawwas et al. (2012)20, our strain ratio was significantly higher among patients with pancreatic malignant tumors compared with those with benign masses. Our optimal cut-off point was pretty similar to Iglesias’ cut-off (8 vs. 6). However, the overall performance of this criterion was not so good in our study. Sensitivity of 90% and specificity of 75% are not much better than our rates for EUS-FNA. Applying Iglesias’ cut-off in their sample, Dawwas et al. (2012) evidenced a less favorable accuracy and diagnostic discrimination. We should be careful with the high rates reported by Iglesias-Garcia et al. (2010)10 because all the procedures were performed in just one center by just one highly-experienced endosonographer. The generalization of their results for a clinical scenario deserves further confirmation. Whether methodologies based on artificial neural networks would also improve this process deserves further prospective studies.11
Saftoiu et al. (2010)6 prospectively assessed the accuracy of the combination of CED-EUS and EUS-E to differentiate focal pancreatic masses in 64 patients. The overall accuracy was 83% for CED-EUS, 82% for EUS-E, and 83% for the combined techniques compared to 93% for EUS-FNA. Our accuracy rates were similar to theirs (79% for CED-EUS and 82% for SR-EUS-E). Although similar, these authors used different approaches for CED-EUS (pulsatility and resistive indices) and EUS-E (post-processing imaging analysis). Both rates, theirs and ours, are still suboptimal and their place should probably be reserved for cases with inconclusive EUS-FNA.
CED-EUS and EUS-E are not suitable for replacing EUS-FNA and histological examination. More important, the goals are to determine the subset of patients who could have some benefit of these techniques, to potentially increase the yield of FNA, and maybe to reduce the number of unnecessary biopsies. These modalities should be supportive tools for EUS-FNA. We should highlight in our study the high sensitivity rate (100%) and the high NPV (100%) for hypovascular pattern as a sign of malignancy in the subset of patients with inconclusive EUS-FNA. Those are the patients who most benefit from CED-EUS. Therefore this technique could assist physicians in making decisions between surgery and follow-up when the biopsy is inconclusive. A hypovascular pattern in a pancreatic mass with an inconclusive EUS-FNA could point to surgery or to repeat the EUS-FNA. On the other hand, a hypervascular pattern in combination with a negative EUS-FNA could point to follow-up.
The strength of this study may be a direct comparison between CED-EUS and SR-EUS-E in the same session because most studies on these new imaging modalities are single arm with possible selection bias. It is a relatively small series, but one of the larger studies directly comparing CED-EUS to EUS-E. Other strengths of this study include its prospective design, participations of 4 endosonographers, and lack of in-room cytopathologist.
At this moment, these novel techniques have suboptimal diagnostic performance. They are promising, but prospective and comparative studies are still pending. In their recent meta-analysis, Fusaroli et al. (2012)21 evidenced the predominance of levels of evidence IIb/III studies and the lack of levels of evidence I studies when evaluating elastography and contrast enhanced EUS for solid pancreatic lesions. Future randomized studies with more adequate power will have to establish the real clinical impact of CED-EUS and EUS-E.
In conclusion, our results suggest that the clinical utility of CED-EUS and SR-EUS-E remains questionable. The overall accuracy of CED-EUS and SR-EUS-E was similar and added no gain to EUS-FNA. The presence of hypovascular pattern in CED-EUS was independently predictive of malignant pancreatic lesion. The subset of patients with inconclusive EUS-FNA could benefit from CED-EUS due to the high sensitivity and high NPV of hypovascular pattern for diagnosing malignancy in this scenario.
REFERENCES
- 1.Giovannini M, Seitz JF, Monges G, et al. Fine needle aspiration citology guided by endoscopic ultrasonography: results in 141 patientes. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 2.Raut CP, Grau AM, Staerkel GA, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–26. doi: 10.1016/S1091-255X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 3.Hirooka Y, Goto H, Ito A, et al. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: a preliminary study. Am J Gastroenterol. 1998;93:632–5. doi: 10.1111/j.1572-0241.1998.179_b.x. [DOI] [PubMed] [Google Scholar]
- 4.Becker D, Strobel D, Bernatik T, et al. Echo-enhanced color-and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784–9. doi: 10.1067/mge.2001.115007. [DOI] [PubMed] [Google Scholar]
- 5.Hocke M, Schulze E, Gottschalk P, et al. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–50. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saftoiu A, Iordache S, Gheonea DI, et al. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses. Gastrointest Endosc. 2010;72:739–47. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 7.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: the first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344–48. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 8.Janssen J, Schlörer E, G L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–8. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 9.Fumihide I, Itoi T, Moriyasu F, et al. Usefulness of the Characterization of Tissue Hardness of Pancreatic Mass Using Elastography Endoscopic Ultrasound. - First Trial of the Quantification Using Strain Ratio. Gastrointest Endosc. 2008;67:AB212. [Google Scholar]
- 10.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative Endoscopic Ultrasound Elastography: An Accurate Method for the Differentiation of Solid Pancreatic Masses. Gastroenterology. 2010;139:1172–80. doi: 10.1053/j.gastro.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 11.Saftoiu A, Vilmann P, Gorunescu F, et al. Efficacy of an Artificial Neural Network-Based Approach to Endoscopic Ultrasound Elastography in Diagnosis of Focal Pancreatic Masses. Clin Gastroenterol Hepatol. 2011 Oct 1; doi: 10.1016/j.cgh.2011.09.014. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich CF, Ignee A, Braden B, et al. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–7. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Figueiredo FAF, Giovannini M, Monges G, et al. Pancreatic Endocrine Tumors: A Large Single-Center Experience. Pancreas. 2009;38:936–40. doi: 10.1097/MPA.0b013e3181b365db. [DOI] [PubMed] [Google Scholar]
- 14.Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–50. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: results of a pilot study. Endoscopy. 2010;42:564–70. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 16.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–7. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 17.Hocke M, Dietrich CF, A.S The Use of Contrast Enhanced Endoscopic Ultrasound in Discrimination Between Focal Pancreatitis and Pancreatic Cancer. Gastrointest Endosc. 2008;67:AB200. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritscher-Ravens A. Blue clouds and green clouds: virtual biopsy via EUS elastography? Endoscopy. 2006;38:416–7. doi: 10.1055/s-2006-925277. [DOI] [PubMed] [Google Scholar]
- 19.Saftoiu A, Vilmann P, Ciurea T, et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 20.Dawwas M, Taha H, Leeds J, et al. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: a prospective, singlecenter study. Gastrointest Endosc. 2012. article in press. available at http://dx.doi.org/10.1016/j.gie.2012.05.034 . [DOI] [PubMed]
- 21.Fusaroli P, Kypraios D, Caletti G, et al. Pancreatico-biliary endoscopic ultrasound: A systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243–56. doi: 10.3748/wjg.v18.i32.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]


