Abstract
Lung cancer is one of the most common tumors worldwide. Pulmonary lesions detected during screening for lung cancer need to be evaluated further and tissue should be obtained. Bronchoscopy is often the first step to secure a histological diagnosis. Differ-ent guidance techniques are available to increase the diagnostic yield. Over the last few years endobronchial ultrasound (EBUS) has been used increasingly to direct the sampling tools, often in combination with a guide sheath. This article offers a review of the literature of the use of EBUS in diagnosis of pulmonary peripheral lesions.
Keywords: bronchoscopy, solitary pulmonary nodule, endobronchial ultrasound, guide sheath
INTRODUCTION
Lung cancer now ranks as the leading cause of cancer related death in the world, both in men and women. The cancer is usually diagnosed in the advanced stages of the disease and screening for the disease has proven difficult.1 If discovered in the early stages, lung cancer may undergo curative resection. The aim has hence been to develop appropriate screening strategies to diagnose this disease earlier.
In the recent National Lung Screening Trial2, patients underwent screening with either regular chest radiographs or low-dose computed tomography (CT). The regular screening with the aid of low dose CT was able to show a reduction in mortality of 20.6% by means of detecting lung cancer in the earlier stages.
It became apparent however that extensive screening reveals pulmonary lesions that require further investigation, despite the majority of these being benign. If screening is to be widely accepted and adopted, these lesions will pose a diagnostic dilemma with regards to the management of these patients.
SOLITARY PULMONARY LESIONS
Solitary pulmonary lesions are defined as parenchymal lesions of less than 3 cm in diameter, not associated with atelectasis or adenopathy, surrounded by normal lung parenchyma. The possibility of malignancy increases with the lesion size. Lesions below 8 mm in size have a low likelihood of malignancy and CT follow-up is recommended in the initial stages. The larger the size of the lesion, the higher the probability of malignancy. Surgical resection may be the treatment of choice for a solitary nodule; however, patients are often elderly or have several comorbidities rendering them unsuitable for a surgical approach. The incidence of solitary pulmonary nodules (SPN) is increasing particularly with the increasing use of screening methods.
In order to obtain a histological diagnosis, transthoracic needle aspiration (TTNA) of these lesions can be attempted when they are located within the periphery of the lung. The procedure is well established and a sensitivity of between 74% and up to 96% has been quoted. But transthoracic needle biopsy carries a high risk of periprocedural pneumothorax, quoted to be between 15% and 44%.3,4,5
Bronchoscopy has been used for many years to evaluate SPN as well as peripheral tumors of the lung. Transbronchial forceps biopsy (TBB) under fluoroscopy is the standard approach to obtain tissue samples of the SPN (Fig. 1). Again, the diagnostic yield is dependent on the size of the lesion, and increases with increasing lesion size as well as visibility under fluoroscopy. For lesions between 2.5 cm and 4.0 cm the sensitivity is described at 62%, whereas for lesions under 2.5 cm it drops to 40%.1,6
Figure 1.
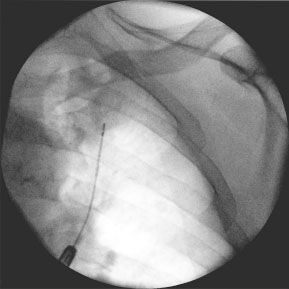
Pulmonary lesion under fluoroscopy with biopsy forceps.
Complications are rare and pneumothoraces are much less frequent after TBB than after TTNA. The use of fluoroscopy for transbronchial biopsy results in radiation exposure for the patients as well as the staff. Some lesions cannot be visualized under fluoroscopy and therefore cannot be biopsied. The size and location of the lesion influence the diagnostic accuracy of TBB under fluoroscopy.6,7
In order to reach peripheral lung nodules more easily and improve the diagnostic yield, different guidance techniques have been developed over the last few years.
ENDOBRONCHIAL ULTRASOUND (EBUS) FOR PERIPHERAL LESIONS
In the early 1990s, a miniaturized ultrasound probe was developed for the use within the airways. Ultrasound probes, so-called miniprobes, with a diameter of 1.4 mm and 1.7 mm are available for the use in the periphery of the lung. These can be advanced via the working channel of a bronchoscope and offer a 360 degree view of surrounding structures. Most commonly a frequency of 20 MHz is used within the lung. The difference in impedance between normal lung tissue and pulmonary lesions has made it an invaluable tool for their detection. Ultrasound is completely reflected by surrounding air within the lung, giving the impression of a snowstorm picture (Fig. 2). Once the miniprobe is within a pulmonary lesion, the picture changes and allows for detailed imaging of the lung lesions. Solid tumors tend to appear darker and have a bright border differentiating them from the surrounding lung tissue (Fig. 3A, B). Ultrasound images of inflammatory tissue or atelectasis have a more inhomogeneous pattern caused by the different structures within the lung, and fluid appears dark. Once the lesion has been reached by the ultrasound probe, the probe is retracted prior to inserting a biopsy forceps through the working channel.
Figure 2.
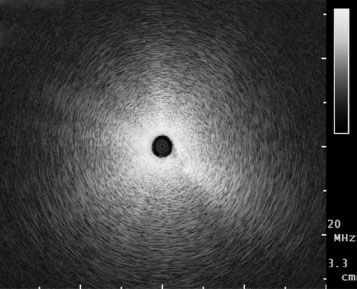
‘Snowstorm’ picture in normal aerated lung.
Figure 3.
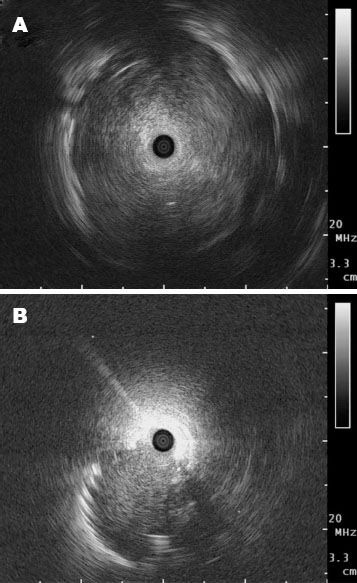
Ultrasound images when a miniprobe is used. A: In the lesion; B: Adjacent to the lesion.
Herth et al8 used the radial EBUS probe initially in 2002 for the detection of lung nodules in the periphery and to guide transbronchial biopsies. This prospective trial compared fluoroscopy-guided versus EBUS-guided transbronchial biopsies showing a non-significant trend for EBUS to be better than fluoroscopy.
The position of the radial EBUS probe in relation to the lesion can be determined as central (within the target) and adjacent (see pictures) to the lesion.
EBUS has significantly increased the yield diagnostic yield of peripheral pulmonary lesions. Diagnostic sensitivities of using radial EBUS for peripheral nodules have been reported to be in the region of 61%-80% independent of lesion size. The procedure is however operator and expertise dependent. If fluoroscopy is used in combination with the miniprobe, the yield can be further improved.
Lesions of less than 30 mm in diameter are often not visible under fluoroscopy. A prospective study assessed the diagnostic yield of these lesions with EBUS guidance. Eighty percent of lesions less than 22 mm in diameter could be localized with EBUS, and in 70% a diagnosis was established with transbronchial biopsy.9 Hence, EBUS allows for image guidance as an alternative to fluoroscopy.
GUIDE SHEATH
In order to improve the yield of EBUS-guided biopsies further, the use of the guide sheath for the radial probe was first introduced by Kurimoto in 2004.10 The procedure is similar to that with the naked radial probe, the probe however is advanced within the guide sheath through the working channel of the flexible bronchoscope (Fig. 4). A standard bronchoscope with a working channel of 2.0 mm can be used together with a guide sheath of 1.9 mm and a 1.4 mm ultrasound probe. If the larger radial probe of 1.7 mm is used, a bronchoscope with a working channel of 2.8 mm needs to be employed. The picture obtained with the radial EBUS probe confirms the position within the lesion and can then be retracted. If the lesion cannot be located in this fashion, a double hinged curette can be inserted into the guide sheath. In this way the appropriate bronchus can now be selected under fluoroscopy and once it has been determined, the curette is removed and the ultrasound reinserted into the guide sheath to confirm the correct position.
Figure 4.

EBUS miniprobe and guide sheath. EBUS: endobronchial ultrasound.
The guide sheath remains within the lesion and now acts as an extended working channel and samples can be taken with forceps, brush or curette. Ideally five biopsies should be taken from the site to obtain a high sensitivity as quoted in a retrospective analysis.11
Use of a guide sheath avoids the need for concurrent fluoroscopy if the lesion can be detected with radial EBUS.
Yoshikawa et al.12 found that EBUS could be used as guidance for transbronchial biopsies with a guide sheath relinquishing the need for fluoroscopy. 61.8% of lesions in his study were diagnosed by the use of EBUS alone. The diagnostic yield in this study was significantly higher for lesions of > 20 mm than for those < 20 mm. The lesion size is certainly a determining factor in being able to visualize them by EBUS as shown in previous studies.13 Even for lesions of less than 20 mm in diameter a definitive diagnosis can be established in 46% patients as shown in a recent study by Eberhardt et al.14
DISCUSSION
Lung lesions continue to pose a diagnostic dilemma. Transbronchial biopsy under fluoroscopy has been the standard for many years. The introduction of radial EBUS has improved diagnostics significantly. Biopsy with the guidance of a radial ultrasound probe has the limitation of not being ‘real time’ as the ultrasound probe has to be removed prior to inserting the forceps via the working channel. The biopsy instrument can sometimes not be reinserted into the same subsegment as the radial EBUS probe and hence biopsies might be from a different area. A guide sheath can overcome this problem by remaining at the position where the lesion was identified with the radial EBUS probe.
Use of a guide sheath however has its own limitations. It limits the size of the biopsy forceps due to the limitations of the working channel. This further means the reduced size of the biopsies due to smaller instruments. The guide sheath bends easily and can prevent the insertion of the biopsy forceps or brush.
A meta-analysis performed by Steinfort et al.15 in 2011 found a pooled sensitivity of 73% and a specificity of 100% for the use of radial EBUS with or without a guide sheath in transbronchial biopsies. They further pointed out, however, that a non-diagnostic result in EBUS-guided TBB should strongly be considered for further investigation to rule out malignancy. In most of the studies examined in this metaanalysis there was no influence of lobar position on the diagnostic sensitivity. Radiological findings on CT can aid to predict the sensitivity of EBUS-guided TBB, e.g., the presence of a bronchus sign that may predict the probability of malignancy of the lesion.
A retrospective trial by Yamada et al11 showed that the diagnostic yield is higher if the miniprobe is central in the lesion than if it is adjacent to the lesion.
Recently, thin bronchoscopes were developed in order to be passed into the periphery more easily, hence negating the need for a guide sheath. Oki et al.16 published a randomized trial comparing the use of an EBUS probe via a thin bronchoscope to using it within a guide sheath. An Olympus prototype videobronchoscope was used with an external diameter of 3.4 mm and a 1.7-mm working channel. Two hundred and three patients were randomized to receiving biopsy of their lesion either with the EBUS guide sheath or via the thin bronchoscope. The diagnostic yield of the thin bronchoscope was non-inferior to the guide sheath method and the procedure time was significantly shorter in the thin- bronchoscope group. The use of ultrathin bronchoscopes has also meant a further reduction of the size of the working channel.
Other navigation techniques such as virtual bronchoscopy and electromagnetic navigation bronchoscopy are additional tools to guide the biopsy of peripheral lesions.
Virtual bronchoscopy is a CT-based imaging technique allowing for noninvasive evaluation of the bronchial tree. It creates a 3-dimensional image of the bronchial tree and a generated map can lead the way to the peripheral lesion, indicating where the bronchoscope should be inserted at each subdivision. The combination of virtual bronchoscopy with EBUS guidance has been demonstrated to be safe, feasible and effective by Asahina et al.17 The diagnostic sensitivity reached 44.4% for lesions < 20 mm and 91.7% for lesions of 20-30 mm.17
Electromagnetic navigation-guided diagnostic bronchoscopy (ENB) allows for guidance of the bronchoscope to the peripheral lesion via a reconstructed CT image in 3-dimension. It is possible to navigate the bronchoscope with this system to invisible lesions but is based on a virtual environment. The lesion cannot be visualized in itself prior to biopsy. The diagnostic sensitivity with this technique was shown to be 67%.18 ENB can also be used in combination with EBUS and the diagnostic sensitivity can be further increased to 88%,19 greater than EBUS or ENB alone. The set-up however is expensive and may not be as readily available in bronchoscopy suites as an ultrasound processor.
Overall, the EBUS guide sheath for transbronchial biopsies and brushings is useful to confirm the precise location of the lesion even if the lesion is not visible under fluoroscopy. A guide sheath aids to obtain the samples from the most adequate position closest to real-time procedure by leaving the guide sheath in place, and bleeding is reduced by trapping the guide sheath in the relevant bronchus.
The combination of several guidance techniques together with the improvement in biopsy tools has improved the diagnostic yield to be closer to the sensitivity achieved with CT-guided (92%)20 or surgical biopsies.
REFERENCES
- 1.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123:115S–28S. doi: 10.1378/chest.123.1_suppl.115s. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines. Chest. (2nd edition) 2007;132:94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Krishnamurthy S, Broemeling LD, et al. Small (</=2-cm) subpleural pulmonary lesions: short- versus long-needle-path CT-guided Biopsy--comparison of diagnostic yields and complications. Radiology. 2005;234:631–7. doi: 10.1148/radiol.2342031423. [DOI] [PubMed] [Google Scholar]
- 5.Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med. 2011;155:137–44. doi: 10.1059/0003-4819-155-3-201108020-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasparini S, Ferretti M, Secchi EB, et al. Integration of transbronchial and percutaneous approach in the diagnosis of peripheral pulmonary nodules or masses. Experience with 1,027 consecutive cases. Chest. 1995;108:131–7. doi: 10.1378/chest.108.1.131. [DOI] [PubMed] [Google Scholar]
- 7.Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest. 2000;117:1049–54. doi: 10.1378/chest.117.4.1049. [DOI] [PubMed] [Google Scholar]
- 8.Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J. 2002;20:972–4. doi: 10.1183/09031936.02.00032001. [DOI] [PubMed] [Google Scholar]
- 9.Herth FJ, Eberhardt R, Becker HD, et al. Endobronchial ultrasound-guided transbronchial lung biopsy in fluoroscopically invisible solitary pulmonary nodules: a prospective trial. Chest. 2006;129:147–50. doi: 10.1378/chest.129.1.147. [DOI] [PubMed] [Google Scholar]
- 10.Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest. 2004;126:959–65. doi: 10.1378/chest.126.3.959. [DOI] [PubMed] [Google Scholar]
- 11.Yamada N, Yamazaki K, Kurimoto N, et al. Factors related to diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in small peripheral pulmonary lesions. Chest. 2007;132:603–8. doi: 10.1378/chest.07-0637. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa M, Sukoh N, Yamazaki K, et al. Diagnostic value of endobronchial ultrasonography with a guide sheath for peripheral pulmonary lesions without X-ray fluoroscopy. Chest. 2007;131:1788–93. doi: 10.1378/chest.06-2506. [DOI] [PubMed] [Google Scholar]
- 13.Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology. 2009;14:859–64. doi: 10.1111/j.1440-1843.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- 14.Eberhardt R, Ernst A, Herth FJ. Ultrasound-guided transbronchial biopsy of solitary pulmonary nodules less than 20 mm. Eur Respir J. 2009;34:1284–7. doi: 10.1183/09031936.00166708. [DOI] [PubMed] [Google Scholar]
- 15.Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37:902–10. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 16.Oki M, Saka H, Kitagawa C, et al. Randomized study of endobronchial ultrasound-guided transbronchial biopsy: thin bronchoscopic method versus guide sheath method. J Thorac Oncol. 2012;7:535–41. doi: 10.1097/JTO.0b013e3182417e60. [DOI] [PubMed] [Google Scholar]
- 17.Asahina H, Yamazaki K, Onodera Y, et al. Transbronchial biopsy using endobronchial ultrasonography with a guide sheath and virtual bronchoscopic navigation. Chest. 2005;128:1761–5. doi: 10.1378/chest.128.3.1761. [DOI] [PubMed] [Google Scholar]
- 18.Eberhardt R, Anantham D, Herth F, et al. Electromagnetic navigation diagnostic bronchoscopy in peripheral lung lesions. Chest. 2007;131:1800–5. doi: 10.1378/chest.06-3016. [DOI] [PubMed] [Google Scholar]
- 19.Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:36–41. doi: 10.1164/rccm.200612-1866OC. [DOI] [PubMed] [Google Scholar]
- 20.Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–54. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]


