Abstract
Ectopic pancreas is the relatively uncommon presence of pancreatic tissue outside the normal location of the pancreas. We report a case of abdominal pain due to retroperitoneal neuroendocrine tumor arising from heterotopic pancreatic tissue between the duodenal wall and the head of the pancreas. Patient underwent surgical enucleation of the tumor.
Keywords: ectopic pancreas, neuroendocrine tumor, endoscopic ultrasonography, retroperitoneal tumor
INTRODUCTION
Ectopic pancreas (EP) is the relatively uncommon presence of pancreatic tissue outside the normal location of the pancreas. This condition is usually asymptomatic and rarely complicated by pancreatitis or malignant transformation. It is defined as pancreatic tissue abnormally situated, without connection to the normal pancreas, but provided with its own vascular and ductal systems.1 EP tissue has been found in both abdominal and extra abdominal locations, but is most frequently encountered in the stomach (25%-60%),2 duodenum (25%-35%)2 and rarely in mesocolon3,4 and Meckel's diverticulum.5 Other terms such as heterotopic and aberrant pancreas have also been described in the literature.6 Retroperitoneal, EP tissue appearing as bilateral suprarenal masses has been reported once in 20097 but a neuroendocrine tumor (NET) in a retroperitoneal EP tissue has not been reported before. Gaspar Fuentes et al. modified von Heinrich's classification of pancreatic heterotopias.8,9 Type I was total pancreatic heterotopia with all pancreatic cell types present, Type II is composed of pancreatic ducts only (the so called canalicular variety), Type III comprises acinar tissue only (exocrine pancreas) and Type IV is made up of islet cells only (endocrine pancreas). The histological classification of the heterotopic pancreas in our case is Type I because all elements were present, although the epithelial component dominated and less frequently the ductal component.
The origin of EP tissue is unknown; it is possible that during rotation of the foregut and fusion of the ventral and dorsal parts of the pancreas in early fetal life, small pieces of tissue become detached from the forming organ leading to entrapment in different locations.10
CASE REPORT
A 72-year-old male was presented by dull aching epigastric and right hypochondrial pain radiating to the back. He is not known to be diabetic or hypertensive. His pulse rate and blood pressure were normal. The physical examination was unremarkable, with no palpable abdominal masses. Lab investigations were normal.
Upper endoscopy revealed a smooth bulge at the mid second part of the duodenum with normal underlying mucosa, likely due to compression from outside. Abdominal ultrasound and computed tomography (CT) showed a well-defined right lumber mass in-between the second part of the duodenum and the head of the pancreas, but appears separated from them. Common bile duct and pancreatic duct are not dilated. No lymphadenopathy was found. Endoscopic ultrasound (EUS) showed a localized paraduodenal echogenic mass measuring 58 mm × 67 mm with a well-defined line of separation form the duodenal wall and the head of the pancreas (Fig. 1). It shows elasticity score 411 denoting its firm consistency (Fig. 2). EUS-guided fine needle aspiration (EUS-FNA) and cell-block preparation was carried out. Histopathological examination revealed the presence of compact groups of epithelial cells having central rounded nuclei with occasional presence of nucleoli and moderate eosinophilic granular cytoplasm separated by delicate stroma showed many small vascular spaces. Immunohistochemistry revealed positive immunostaining for synaptophysin and chromogranin (Fig. 3). The decision for surgical intervention was taken. On laparotomy, a retroperitoneal well encapsulated mass was found between the wall of the duodenum and the head of the pancreas and separated from them. Enucleation of the tumor was performed and pathological examination confirmed the FNA diagnosis.
Figure 1.
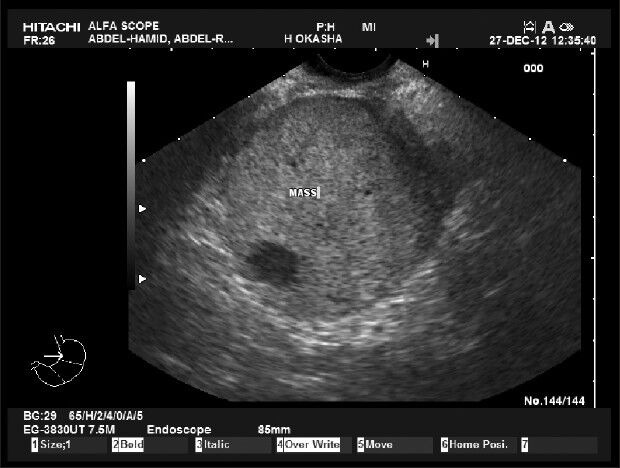
Endoscopic ultrasound picture of the mass
Figure 2.
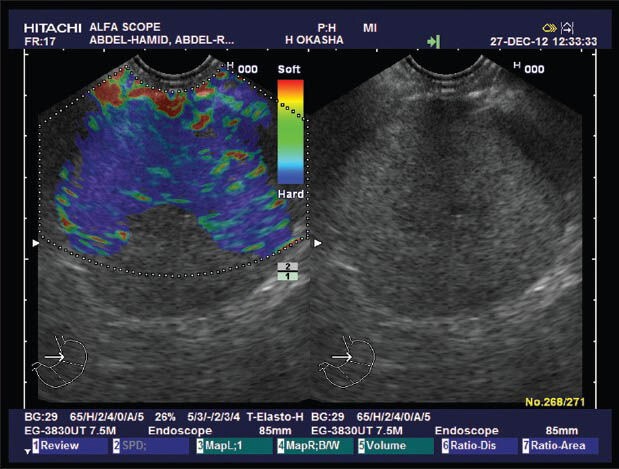
Elastography of the mass (elasticity score 4)
Figure 3.
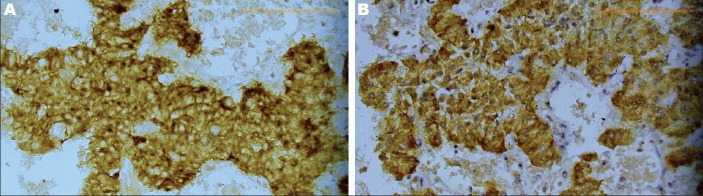
Tumor cells showing positive immunostaining for chromogranin (A) and synaptophysin (B) (×400)
DISCUSSION
Several theories had been implicated to describe the origin of EP tissue. The most accepted one implicates migration and/or rests of branching pancreatic tissue buds from the developing pancreas during embryogenesis. This theory is most likely when one considers gastrointestinal pancreatic heterotopia; however, pancreatic tissue occurring in more exotic, anatomically remote sites, such as the thyroid gland or fallopian tube, may have a different pathogenesis. The possibility of origin from teratomas has been advocated in these instances.
The pancreatic ectopic tissue is usually presented by gastric pain and patients may also complain of bleeding, nausea and vomiting and chest pain. The mechanism for the production of symptoms is unclear. Armstrong et al. found a correlation between the presence of symptoms, size of the lesion (greater than 1.5 cm) and extent of mucosal involvement.12
EP typically appears on a radiographic barium study or endoscopic examination as a submucosal mass with central umbilication.13,14 On CT, an EP most frequently appears as a well-defined oval or round mass with smooth or serrated margins in the gastric antral wall or intestinal wall or as a mesenteric mass similar to a gastrointestinal stromal tumor or carcinoid tumor.13,14,15
EUS is typically used to evaluate the submucosal lesions in the upper gastrointestinal tract.16 EP usually appears hypoechoic and heterogeneous with indistinct margins during EUS and the most commonly originates from the third or fourth layer or a combination of these two layers of the gastrointestinal tract.17 EUS also allows the use of targeted FNA biopsy, particularly helpful for the final diagnosis. Although cytological examinations are inconclusive in about 50% of cases,10 in this case EUS-FNA gave the same result as the excisional biopsy as being NET.
We report, to our knowledge, the first case of a NET in a retroperitoneal EP tissue. Heterotopic pancreas should be considered in the differential diagnosis of a retroperitoneal masses as well as a submucosal mass of the gastric, duodenal, jejunal and esophageal wall and it has the same line of management as pancreatic NET, which is surgical resection that should remain the mainstay of treatment for patients with a localized disease.
REFERENCES
- 1.Hsu SD, Chan DC, Hsieh HF, et al. Ectopic pancreas presenting as ampulla of vater tumor. Am J Surg. 2008;195:498–500. doi: 10.1016/j.amjsurg.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Mulholland KC, Wallace WD, Epanomeritakis E, et al. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004;5:498–501. [PubMed] [Google Scholar]
- 3.Ishikawa O, Ishiguro S, Ohhigashi H, et al. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990;85:597–601. [PubMed] [Google Scholar]
- 4.Tornóczky T, Kálmán E, Jáksó P, et al. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol. 2001;54:241–5. doi: 10.1136/jcp.54.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh HC, Page B, Black C, et al. Ectopic pancreatic-type malignancy presenting in a Meckel's diverticulum: A case report and review of the literature. World J Surg Oncol. 2009;7:54. doi: 10.1186/1477-7819-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizuno Y, Sumi Y, Nachi S, et al. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today. 2007;37:704–7. doi: 10.1007/s00595-006-3384-5. [DOI] [PubMed] [Google Scholar]
- 7.Lin LH, Ko SF, Huang CC, et al. Retroperitoneal ectopic pancreas: Imaging findings. Br J Radiol. 2009;82:e253–5. doi: 10.1259/bjr/27696141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspar Fuentes A, Campos Tarrech JM, Fernández Burgui JL, et al. Pancreatic ectopias. Rev Esp Enferm Apar Dig. 1973;39:255–68. [PubMed] [Google Scholar]
- 9.Hammock L, Jorda M. Gastric endocrine pancreatic heterotopia. Arch Pathol Lab Med. 2002;126:464–7. doi: 10.5858/2002-126-0464-GEPH. [DOI] [PubMed] [Google Scholar]
- 10.Tolentino LF, Lee H, Maung T, et al. Islet cell tumor arising from a heterotopic pancreas in the duodenal wall with ulceration. Exp Mol Pathol. 2004;76:51–6. doi: 10.1016/j.yexmp.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini M. Endoscopic ultrasound (EUS) elastography. MEDIX Supplement. Special Issue: Clinical Applications of HITACHI Real-time Tissue Elastography. 2007:32–5. [Google Scholar]
- 12.Armstrong CP, King PM, Dixon JM, et al. The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg. 1981;68:384–7. doi: 10.1002/bjs.1800680606. [DOI] [PubMed] [Google Scholar]
- 13.Mortelé KJ, Rocha TC, Streeter JL, et al. Multimodality imaging of pancreatic and biliary congenital anomalies. Radiographics. 2006;26:715–31. doi: 10.1148/rg.263055164. [DOI] [PubMed] [Google Scholar]
- 14.Silva AC, Charles JC, Kimery BD, et al. MR Cholangiopancreatography in the detection of symptomatic ectopic pancreatitis in the small-bowel mesentery. AJR Am J Roentgenol. 2006;187:W195–7. doi: 10.2214/AJR.04.1756. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Kuo Y, Yeung K, et al. CT appearance of ectopic pancreas: A case report. Abdom Imaging. 1998;23:332–3. doi: 10.1007/s002619900351. [DOI] [PubMed] [Google Scholar]
- 16.Yasuda K, Cho E, Nakajima M, et al. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S17–20. doi: 10.1016/s0016-5107(90)71010-3. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita M, Hajiro K, Okazaki K, et al. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc. 1999;49:493–7. doi: 10.1016/s0016-5107(99)70049-0. [DOI] [PubMed] [Google Scholar]


