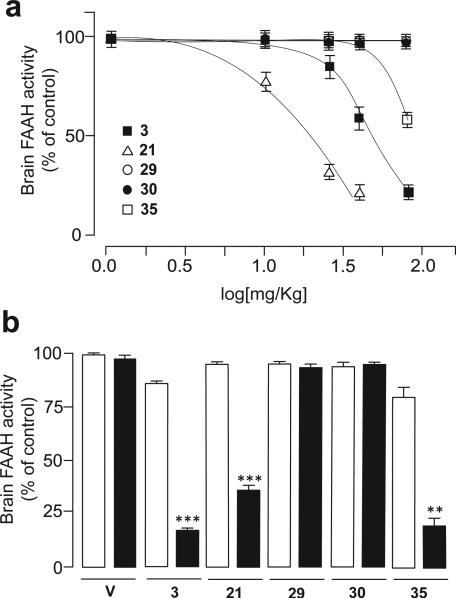
Figure 3.
Inhibition of brain FAAH activity by analogues of compound 3 bearing different substituents on the meta- or para- position of the proximal phenyl ring; a) Dose-dependent inhibition of brain FAAH activity by p-hydroxymethyl (21), p-carboxyl (29), p-sulfate (30) and m-hydroxy (35) derivatives of compound 3 in Swiss Webster mice; doses were 0.3–75 mg/kg (s.c.); b) Effects of pharmacological blockade of the Abcg2 transporter (Ko-143, 15 mg/kg, i.p., closed bars) on brain inhibition of FAAH activity caused by a sub-effective dose (selected from the dose-response study: 3 (25); 21 (10); 29 (40); 30 (75); 35 (40) in mg/kg, s.c., open bars) of analogues of compound 3 bearing different functionalities on the meta- position of the distal phenyl ring. Results are expressed as mean ± s.e.m. (n = 3-4). *** P< 0.001, ** P< 0.01 vs non-Ko-143 treated group.