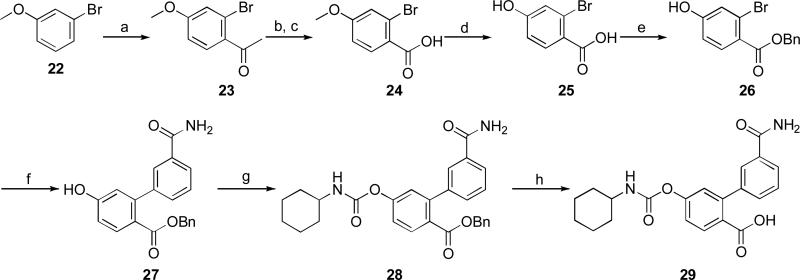
Scheme 5.
Reagents and conditions: a) AcCl, ZrCl4, DCM, 0 °C to rt, 1 h, 55%; b) diethyl oxalate, t-BuONa, THF, rt, 30 min, 77%; c) Oxone®, acetone, 0 °C, 2 h, 61%; d) BBr3, DCM, 0 °C to rt, 12h, 61%; e) BnBr, KHCO3, DMF, rt, 12h; f) 3-carbamoylphenylboronic acid, PdCl2dppf, K2CO3, dioxane/H2O, 90 °C, 1 h, 57%; g) c-C6H11NCO, Et3N, CH3CN/EtOH, 45 °C, 12h, 76%; h) 10% Pd/C, cyclohexene, dioxane, 80 °C, 2 h, 44%.