Figure 1.
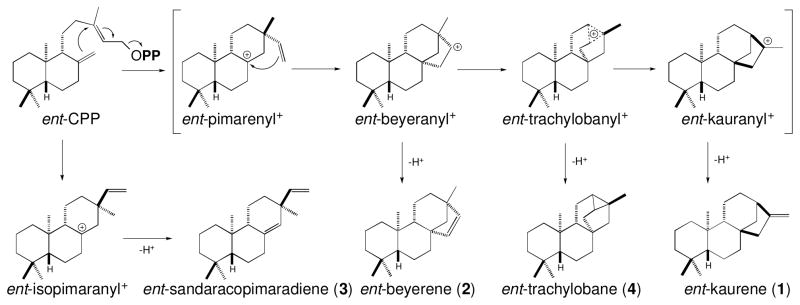
Cyclization mechanisms for the diterpene synthase activities previously identified in castor bean by assays with cell-free extracts (Robinson and West, 1970b; Spickett et al., 1994), and their relationship to the classical mechanism for production of ent-kaurene (1). This classic mechanism proceeds via ionization of the allylic diphosphate ester bond in ent-CPP to trigger initial cyclization to the depicted ent-pimarenyl+ intermediate, followed by secondary cyclization to the depicted ent-beyeranyl+ intermediate that rearranges via the depicted ent-trachylobanyl+ intermediate en route to the ent-kauranyl+ intermediate that is quenched by deprotonation to yield ent-kaurene. As shown, the production of ent-beyerene (4) and ent-trachylobane (2) similarly arises from deprotonation of the corresponding carbocations.
