Figure 2.
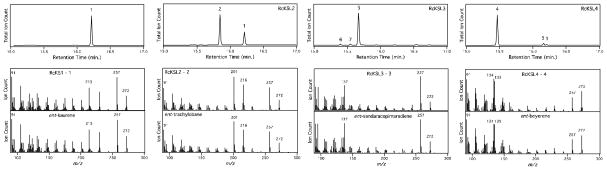
Identification of products formed by the RcKS(L)s by comparison to authentic standards by GC-MS. Chromatographs of the products formed from ent-CPP by corrected RcKS1, RcKSL2, RcKSL3 and corrected RcKSL4, as indicated (with corrections as described in the text). Peaks are labeled with numbering corresponding to that used in the text (with structures shown in Figures 1 and/or 5), along with ent-labdatriene (6) and ent-pimaradiene (7). Also shown are mass spectra of the major enzymatic products, their retention times (RT) were 16. 22 min. for RcKSL1; 15.84 min. for RcKSL2; 15.67 min. for RcKSL3; and 15.46 min. for RcKSL4, along with those from the corresponding authentic standards [RT = 16.23 min. for ent-kaurene (1); 15.85 min. for ent-trachylobane (2); 15.67 min. for ent-sandaracopimaradiene (3); and 15.47 min. for ent-beyerene (4)].
