Abstract
Background
Observational studies comparing neoadjuvant chemotherapy to primary surgery for advanced-stage ovarian cancer are limited by strong selection bias. We used multiple methods to control for confounding and selection bias to estimate the effect of primary tretment on survival for ovarian cancer.
Methods
The Surveillance, Epidemiology, and End Results (SEER)-Medicare database was used to identify women ≥65 years of age with stage II-IV epithelial ovarian cancer who survived >6 months from the date of diagnosis and received treatment from 1991-2007. Traditional regression analysis, propensity score-based analysis, and an instrumental variable analysis (IVA) using geographic location as an instrument were used to compare survival between neoadjuvant chemotherapy and primary surgery.
Results
A total of 9587 patients with stage II-IV ovarian cancer were identified. Use of primary surgery decreased from 63.2% in 1991 to 49.5% by 2007, while primary chemotherapy increased from 19.7% in 1991 to 31.8% in 2007 (P<0.0001). In the observational cohort survival (HR=1.27; 95% CI, 1.19-1.35) was inferior for patients treated with neoadjuvant chemotherapy; both median survival (15.8 vs. 28.8 months) and two-year survival (36% vs. 56%) were lower in the neoadjuvant chemotherapy group compared to those who underwent surgery. In the IVA, primary treatment had minimal effect on overall survival (HR=1.04; 95% CI, 0.67-1.60). The median survival for patients with a value of the instrument less than the median (24.0 months, 95% CI, 23.0-25.0) and ≥median value of the IV (24.0 months, 95% CI 23.0-26.0) were similar.
Conclusion
Use of neoadjuvant therapy has increased over time. Survival with neoadjuvant chemotherapy did not differ significantly from primary surgery in elderly women in the U.S.
Introduction
Conventional treatment for ovarian cancer relies on surgical cytoreduction followed by adjuvant chemotherapy. 1,2 Given the morbidity of cytoreductive surgery, neoadjuvant chemotherapy followed by interval surgery has been proposed as an alternative treatment strategy.3-8 Recently, a randomized controlled trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) of over 600 women found that survival was similar for the two strategies while morbidity was lower in those who received neoadjuvant chemotherapy.3 While promising, concern has been raised that the rate of tumor resection to minimal disease volume was low in the primary surgical arm and that survival, in both arms, was inferior to that reported in many contemporaneous studies.9,10
A further concern regarding neoadjuvant chemotherapy stems from the results of published observational studies.5,6 Many of these reports have suggested that survival for neoadjuvant chemotherapy is inferior to primary surgery or, at best, similar to survival after suboptimal tumor cytoreduction.5,6 A major limitation of these reports is the substantial underlying selection bias in the allocation of upfront treatment; those women with the worst prognosis are the most likely to receive neoadjuvant therapy.4,8
Observational studies have typically used regression-based methodology to account for differences between treatment groups, however traditional regression methods are unlikely to completely correct for selection bias and cannot account for the effects of unmeasured confounders on outcome.11,12
To overcome these limitations, a number of statistical techniques for the analysis of observational data have been developed.11-16 Unlike regression analysis that adjusts for the effect of confounding variables on an outcome, propensity score analysis estimates the probability that a patient will undergo a given treatment or intervention.12,15 The resulting propensity score is then used to match patients or as a variable for further modeling.12,15,16 An instrumental variable analysis (IVA) is a statistical methodology developed to account for the effects of both measured and unmeasured confounders on outcome.11-14 An IVA uses an exogenous variable or instrument, such as distance from treatment center, which is correlated with treatment choice but not outcome. Variations in the value of the instrument that are associated with variation in treatment choice can be used to overcome measured and unmeasured confounding.11-14
Given the limitations of prior studies using observational data comparing the outcomes of treatment for ovarian cancer, we performed a population-based analysis to examine the effectiveness of upfront treatment strategies for elderly women with epithelial ovarian cancer. We estimated the effect of neoadjuvant chemotherapy and primary surgery on survival and compared the results obtained from traditional regression-based risk adjustment to propensity score analysis and an instrumental variable analysis leveraging geographic variations in treatment.
Methods
Data
The Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database was analyzed. SEER is a population-based cancer registry that provides data on tumor characteristics and survival, as well as demographic data. The Medicare database includes data on patients with Medicare part A (inpatient) and part B (outpatient) including billed claims and services.17 Exemption from the Columbia University Institutional Review Board was obtained.
Clinical and demographic characteristics
Women with epithelial ovarian cancer diagnosed between January 1, 1991, and December 31, 2007 were analyzed. Women ≥65 years of age with stage II-IV neoplasms were included.18,19 They were classified into three groups based on their initial treatment: primary surgery, primary chemotherapy, or no treatment.20,21 Upfront treatment was considered as therapy that was initiated within 12 months of the date of diagnosis. Those in the primary surgery group underwent cancer-directed surgery as initial treatment while those in the primary chemotherapy stratum received cytotoxic chemotherapy first. Patients with a claim for surgery and chemotherapy on the same date were classified in the primary surgery group. We excluded patients who were enrolled in a non-Medicare health maintenance organization, those enrolled in Medicare because of end-stage renal disease and dialysis, and patients with other primary tumors.17 A priori the goal of our analysis was to compare outcomes of patients who underwent therapy (either surgery followed by chemotherapy or vice versa) with a goal of extending survival, not merely as palliation of symptoms. We therefore only included patients who survived for more than 6 months after diagnosis.
Age at diagnosis was classified into 5-year intervals. The SEER marital status variable was recorded as married, not married, and unknown. An aggregate socioeconomic status (SES) score was generated from education, poverty level, and income data from the 2000 census tract data.22 The prevalence of comorbid disease in the cohort was estimated using the Klabunde adaptation of the Charlson comorbidity index (i.e., the Klabunde–Charlson index).23,24 Area of residence was categorized as metropolitan or nonmetropolitan and each patients registry recorded. Tumor grade was stratified as well, moderately, or poorly differentiated or unknown, and histology classified as serous, mucinous, endometrioid, clear cell, or other.
Statistical analysis
Frequency distributions between categorical variables were compared using χ2 tests. Multivariable logistic regression models were developed to examine predictors of primary chemotherapy and no treatment. These models contained all of the clinical and demographic characteristics of interest including age, year of diagnosis, race, marital status, SES, area of residence, SEER registry, grade, histology, comorbidity, and stage. For the survival analysis, only patients who initiated some form of treatment (either chemotherapy or surgery) were included. Overall survival was estimated from the date of diagnosis until death from any cause while cancer-specific survival was defined as the period from diagnosis until death from cancer. A second analysis of intended treatment was performed. Intended treatment was defined as receipt of at least one cycle of postoperative chemotherapy in the patients in the primary surgical arm and as surgery after chemotherapy in those women in the primary chemotherapy arm.
For each outcome, survival was compared using multivariable regression analysis, propensity score matching, propensity score matching using inverse probability of treatment weights, and an instrumental variable analysis. The regression-based estimation of survival was performed using multivariable Cox proportional hazards models.
A propensity score is the predicted probability that a subject will undergo a treatment of interest, in the case of the current study, primary chemotherapy for ovarian cancer.11,12,15 A logistic regression model that included all of the clinical and demographic characteristics (age, year of diagnosis, race, marital status, SES, area of residence, registry, grade, histology, stage, comorbidity) of patients who received treatment was constructed to determine the probability of receipt of primary chemotherapy. For each patient, a predicted probability (the propensity score) that ranged from 0 to 1 was generated. The propensity score was first used to perform a propensity score-based match.15 Using a matching algorithm with a caliper of 0.005, we performed a 1-to-1 match for patients who underwent primary surgery to those who had primary chemotherapy.15 Several sensitivity analyses were performed matching different numbers of cases to controls and applying varying caliper settings.
The second propensity score-based methodology used the inverse probability of treatment weighting approach (IPTW).11,16 Using a weighting approach each patient is assigned a differential weight based on their propensity. The IPTW methodology allows inclusion of all patients from the original analysis and does not require a match. The weighting assumptions based on the IPTW methods used assigned patients in the primary chemotherapy arm a weight of 1/propensity score and women in the primary surgery cohort a weight of 1/(1-propensity score).11,16
Instrumental variable analyses attempt to adjust for both measured and unmeasured characteristics through use of an exogenous instrument.11-13 The instrument in these analyses is some characteristic that is associated with treatment but not outcome. Because of variation in the value of the instrument, which approximates randomization, patient groups should have similar observed and unobserved characteristics.11 While a variety of instruments have been described, geographic variation in treatment patterns is commonly chosen.11
Geographic variation in the upfront treatment of ovarian cancer was the instrument chosen for analysis. The primary geographical unit was The Dartmouth Atlas of Health Care's hospital referral regions (HRR). An HRR is a geographic region that represents a healthcare market that generally requires a major referral center.25 Using each patient's county of residence we assigned all subjects within the dataset to an HRR. Patients residing in hospital referral regions with fewer than 25 cumulative subjects during the study period were deleted (n=189). Similarly, some contiguous hospital referral regions were combined to ensure that each HRR included at least 25 patients that received some cancer-directed therapy (either primary chemotherapy or surgery).
The instrumental variable was constructed based on the methodology of Hadley et al.11 Initially, a model was developed to determine the predicted probability of receipt of chemotherapy based on all the demographic and clinical characteristics of the cohort. The difference between the observed use of primary chemotherapy and the average predicted probability of receipt of primary chemotherapy for all patients within a given HRR was then determined. This difference between the observed and expected (O-E) use of chemotherapy served as the instrumental variable. Thus, hospital referral regions with a negative value had fewer patients than predicted who were treated with primary chemotherapy while HRR's with a positive value included more patients who received chemotherapy than were predicted. As shown in supplemental table 1, there was substantial geographic variability in upfront treatment (F=686.61, P<0.0001). Survival for the instrumental variable analysis was estimated using the two-stage residual inclusion method.26 All analyses were performed with SAS version 9.3 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided. A P-value of <0.05 was considered statistically significant.
Results
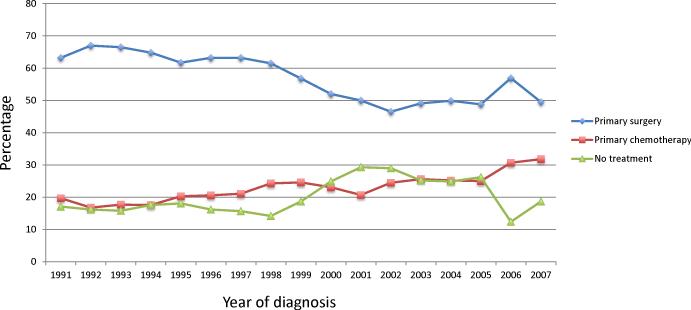
A total of 9587 patients with stage II-IV ovarian cancer including 5345 (55.8%) who underwent primary surgery, 2238 (23.3%) treated with primary chemotherapy and 2004 (20.9%) who received no treatment, were identified (Supplemental Table 1). The number of women who did not undergo treatment remained relatively stable at 17.1% in 1991 compared to 18.7% in 2007 (Figure 1). In contrast, the percentage of women who underwent primary surgery decreased from 63.2% in 1991 to 49.5% by 2007, while the percentage of patients treated primarily with chemotherapy increased from 19.7% in 1991 to 31.8% in 2007 (P<0.0001).
Figure 1.
Upfront treatment for women with ovarian cancer stratified by year of diagnosis.
In a multivariable model, predictors of not receiving treatment included age at diagnosis, year of diagnosis, marital status, area of residence, tumor grade and histology, comorbidity, and stage (Supplemental Table 2). After exclusion of patients who did not receive any treatment, older patients, those diagnosed more recently, women with serous tumors (compared to endometrioid and mucinous carcinomas) and patients who lived in metropolitan areas were more likely to receive primary chemotherapy than surgery. Similarly, patients with a comorbidity score of 1 (OR=1.23; 95% CI, 1.06-1.42) or ≥2 (OR=1.44; 95% CI, 1.20-1.74) compared to 0, and patients with stage III (OR=2.08; 95% CI, 1.59-2.73) and IV (OR=3.72; 95% CI, 2.83-4.89) carcinomas compared to stage II neoplasms were more likely to receive treatment with primary chemotherapy.
There were substantial imbalances between the treatment groups, suggesting strong selection bias in the allocation of primary treatment. After propensity score matching, the only covariate that remained statistically significantly different between the primary chemotherapy and surgery groups was year of diagnosis (Table 1). After propensity score reweighting using the inverse probability of treatment weights, year of diagnosis, area of residence, SEER registry, tumor grade and stage remained imbalanced. Construction of the instrumental variable demonstrated a strong association between area of residence and primary treatment (F=686.61, P<0.0001) (Supplemental Table 3). The variation in the difference between the observed and expected rate of treatment with primary chemotherapy ranged from -16.3% in Santa Cruz, CA to 14.4% in San Diego, CA. To descriptively characterize the cohort based on the instrumental variable, subjects were grouped based on whether the IV for a given patient was below or greater than or equal to the median value of the instrumental variable. Among women with an IV below the median, 26.2% received primary chemotherapy compared to 34.9% for those in the group above the median.
Table 1.
Characteristics of observational, propensity score matched, propensity score inverse probability of treatment weighted (IPTW), and patients grouped by median value of instrumental variable cohort grouped by median value of the instrument.
| Observational | Propensity score matched | Propensity score IPTW | Instrumental variable | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery | CT | P-value | Surgery | CT | P-value | Surgery | CT | P-value | Below | Above | P-value | |
| 5345 | 2238 | 1442 | 1442 | 7356 | 7967 | |||||||
| Age | <0.0001 | 0.91 | 0.41 | 0.72 | ||||||||
| 65-69 | 23.3 | 17.6 | 20.3 | 20.6 | 21.9 | 22.7 | 21.4 | 22.0 | ||||
| 70-74 | 29.1 | 24.9 | 27.1 | 27.4 | 28.4 | 28.5 | 28.2 | 27.3 | ||||
| 75-79 | 26.4 | 26.3 | 27.8 | 26.6 | 27.0 | 27.1 | 26.5 | 26.1 | ||||
| ≥80 | 21.2 | 31.1 | 24.8 | 25.4 | 22.7 | 21.7 | 23.9 | 24.6 | ||||
| Year of diagnosis | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| 1991-1996 | 33.8 | 23.4 | 34.3 | 24.9 | 34.2 | 24.2 | 26.8 | 21.1 | ||||
| 1997-2002 | 34.1 | 35.1 | 33.6 | 34.3 | 34.3 | 35.8 | 34.0 | 35.1 | ||||
| 2003-2007 | 32.1 | 41.5 | 32.1 | 40.8 | 31.6 | 40.0 | 29.2 | 43.9 | ||||
| Race | 0.02 | 0.73 | 0.81 | <0.0001 | ||||||||
| White | 90.9 | 88.7 | 89.5 | 89.7 | 89.9 | 89.8 | 91.0 | 89.0 | ||||
| Black | 4.1 | 5.5 | 5.3 | 4.8 | 4.7 | 4.6 | 4.5 | 4.5 | ||||
| Hispanic | 1.2 | 1.2 | 1.1 | 0.8 | 1.2 | 1.3 | 1.3 | 1.1 | ||||
| Missing/other | 3.8 | 4.6 | 4.2 | 4.7 | 4.2 | 4.3 | 3.2 | 5.4 | ||||
| Marital status | <0.0001 | 0.87 | 0.53 | 0.17 | ||||||||
| Married | 46.8 | 41.7 | 44.2 | 45.2 | 45.7 | 46.3 | 45.3 | 45.3 | ||||
| Unmarried | 50.5 | 56.1 | 53.7 | 52.8 | 51.7 | 51.3 | 52.4 | 51.7 | ||||
| Unknown | 2.8 | 2.2 | 2.2 | 2.1 | 2.7 | 2.4 | 2.3 | 3.0 | ||||
| Socioeconomic status | 0.67 | 0.99 | 0.20 | <0.0001 | ||||||||
| Lowest (first) quintile | 10.5 | 10.0 | 8.5 | 9.2 | 10.2 | 9.3 | 9.6 | 11.6 | ||||
| Second quintile | 17.6 | 19.0 | 17.6 | 17.7 | 18.0 | 17.7 | 17.5 | 18.9 | ||||
| Third quintile | 22.9 | 22.2 | 23.4 | 23.0 | 22.8 | 23.8 | 23.5 | 21.4 | ||||
| Fourth quintile | 23.1 | 23.2 | 23.7 | 23.7 | 23.2 | 22.5 | 24.7 | 20.8 | ||||
| Highest (fifth) quintile | 24.9 | 24.8 | 25.7 | 25.5 | 24.8 | 25.6 | 2.3 | 25.8 | ||||
| Unknown | 1.0 | 0.8 | 1.2 | 1.0 | 1.0 | 1.1 | 0.5 | 1.6 | ||||
| Area of residence | 0.09 | 0.07 | 0.0009 | 0.57 | ||||||||
| Metropolitan | 92.1 | 93.2 | 94.8 | 93.2 | 92.6 | 93.9 | 92.5 | 92.2 | ||||
| Non-metropolitan | 7.9 | 6.8 | 5.2 | 6.8 | 7.4 | 6.1 | 7.5 | 7.8 | ||||
| SEER Registry | <0.0001 | 0.98 | 0.02 | <0.0001 | ||||||||
| Atlanta | 4.6 | 5.2 | 5.8 | 5.1 | 5.0 | 6.2 | - | 12.3 | ||||
| Connecticut | 10.1 | 11.3 | 12.2 | 11.7 | 10.7 | 11.2 | 7.0 | 16.0 | ||||
| Detroit | 12.0 | 11.9 | 11.4 | 10.3 | 12.0 | 12.7 | 19.5 | - | ||||
| Greater California | 10.0 | 13.8 | 12.5 | 12.7 | 10.6 | 10.8 | 2.2 | 25.4 | ||||
| Hawaii | 1.3 | 1.6 | 1.3 | 1.4 | 1.3 | 1.2 | - | 3.6 | ||||
| Iowa | 11.2 | 9.6 | 8.1 | 9.8 | 10.5 | 9.2 | 14.9 | 4.1 | ||||
| Kentucky | 4.0 | 3.6 | 4.0 | 3.7 | 4.1 | 3.8 | 1.3 | 8.1 | ||||
| Los Angeles | 11.4 | 8.9 | 9.5 | 9.5 | 10.7 | 10.9 | 17.3 | - | ||||
| Louisiana | 0.8 | 1.1 | 0.8 | 1.0 | 0.9 | 0.8 | - | 2.3 | ||||
| New Jersey | 10.5 | 12.3 | 11.8 | 11.9 | 10.6 | 10.3 | 10.0 | 12.6 | ||||
| New Mexico | 3.1 | 3.1 | 3.2 | 3.1 | 3.0 | 2.9 | 0.7 | 6.9 | ||||
| Rural Georgia | 0.1 | 0.2 | 0.2 | 0.3 | 0.2 | 0.1 | - | 0.4 | ||||
| San Francisco | 5.5 | 5.1 | 5.3 | 5.9 | 5.8 | 5.5 | 6.3 | 4.0 | ||||
| San Jose | 3.9 | 2.4 | 3.5 | 3.0 | 3.6 | 3.5 | 4.0 | 2.5 | ||||
| Seattle | 7.3 | 6.3 | 6.8 | 7.1 | 7.1 | 7.5 | 10.8 | 0.9 | ||||
| Utah | 4.3 | 3.7 | 3.5 | 3.6 | 4.0 | 3.4 | 6.0 | 1.0 | ||||
| Grade | <0.0001 | 0.78 | <0.0001 | 0.87 | ||||||||
| 1 | 3.7 | 0.9 | 1.1 | 1.5 | 2.9 | 1.6 | 3.0 | 2.7 | ||||
| 2 | 17.5 | 7.9 | 11.5 | 12.0 | 15.1 | 15.2 | 14.5 | 14.8 | ||||
| 3 | 62.9 | 32.4 | 51.4 | 50.1 | 56.2 | 57.2 | 54.0 | 53.9 | ||||
| Unknown | 15.9 | 58.8 | 36.0 | 36.4 | 25.8 | 26.0 | 28.5 | 28.6 | ||||
| Histology | <0.0001 | 0.70 | 0.90 | 0.02 | ||||||||
| Serous | 66.7 | 34.3 | 54.3 | 52.2 | 59.5 | 59.1 | 58.0 | 55.8 | ||||
| Mucinous | 4.9 | 3.5 | 4.7 | 5.3 | 4.6 | 4.4 | 4.7 | 4.2 | ||||
| Endometrioid | 9.2 | 1.7 | 2.4 | 2.7 | 7.2 | 7.5 | 7.1 | 6.9 | ||||
| Clear cell | 2.5 | 1.0 | 1.8 | 1.5 | 2.1 | 2.1 | 2.3 | 1.8 | ||||
| Other/not otherwise specified | 16.7 | 59.5 | 36.8 | 38.4 | 26.6 | 26.9 | 28.1 | 31.4 | ||||
| Comorbidity score | <0.0001 | 0.22 | 0.23 | 0.97 | ||||||||
| 0 | 68.1 | 58.7 | 63.8 | 61.4 | 66.2 | 67.1 | 65.5 | 65.2 | ||||
| 1 | 21.1 | 25.0 | 23.9 | 24.1 | 22.1 | 22.0 | 22.2 | 22.4 | ||||
| ≥2 | 10.8 | 16.3 | 12.3 | 14.4 | 11.7 | 10.8 | 12.3 | 12.5 | ||||
| Stage | <0.0001 | 0.35 | 0.006 | 0.47 | ||||||||
| II | 11.0 | 3.9 | 3.9 | 5.1 | 9.0 | 7.6 | 8.7 | 9.3 | ||||
| III | 54.8 | 39.4 | 45.6 | 45.7 | 51.1 | 51.3 | 50.1 | 50.6 | ||||
| IV | 34.2 | 56.7 | 49.5 | 49.3 | 39.9 | 41.1 | 41.3 | 40.1 | ||||
Data expressed as percentages.
CT=chemotherapy.
Survival was inferior for patients treated with primary chemotherapy in the observational cohort (cancer specific survival HR=1.26; 95% CI, 1.17-1.35, overall survival HR=1.27; 95% CI, 1.19-1.35) (Table 2). In this analysis, both median survival (15.8 vs. 28.8 months) and two-year survival (36% vs. 56%) were lower in the primary chemotherapy group compared to those who underwent surgery (Table 3). Similar findings were noted in both the propensity score matched (overall survival HR=1.24; 95% CI, 1.15-1.34) and IPTW cohorts (overall survival HR=1.30; 95% CI, 1.25-1.35). In the propensity score-based inverse probability weighting, median survival was 27.2 months (95% CI, 26.0- 28.2 months) for patients treated with primary surgery compared to 21.0 months (95% CI, 20.1-21.5 months) for those who received primary chemotherapy. In contrast, in the instrumental variable analysis, primary treatment had minimal effect on either cancer-specific (HR=0.94; 95% CI, 0.58-1.52) or overall (HR=1.04; 95% CI, 0.67-1.60) survival. Based on this methodology, the median survival for patients with a value of the instrument less than the median (24.0 months, 95% CI, 23.0-25.0) and ≥median value of the IV (24.0 months, 95% CI 23.0-26.0) were similar.
Table 2.
Adjusted Cox proportional hazards models comparing survival of primary chemotherapy vs. surgery stratified by analytic method.
| Death from ovarian cancer | All cause mortality | |||
|---|---|---|---|---|
| Adjusted HR (95% CI) | P-value | Adjusted HR (95% CI) | P-value | |
| All patients | ||||
| Observational | 1.26 (1.17-1.35) | <0.0001 | 1.27 (1.19-1.35) | <0.0001 |
| PS reweighted (matched) | 1.25 (1.15-1.36) | <0.0001 | 1.24 (1.15-1.34) | <0.0001 |
| PS reweighted (IPTW) | 1.31 (1.26-1.37) | <0.0001 | 1.30 (1.25-1.35) | <0.0001 |
| Instrumental variable | 0.94 (0.58-1.52) | 0.80 | 1.04 (0.67-1.60) | 0.87 |
| Intended treatment | ||||
| Observational | 1.17 (1.06-1.29) | 0.002 | 1.21 (1.11-1.33) | <0.0001 |
| PS reweighted (matched) | 1.11 (0.99-1.25) | 0.08 | 1.13 (1.02-1.26) | 0.02 |
| PS reweighted (IPTW) | 1.22 (1.16-1.29) | <0.0001 | 1.24 (1.18-1.30) | <0.0001 |
| Instrumental variable | 0.44 (0.24-0.84) | 0.01 | 0.57 (0.32-1.01) | 0.05 |
Table 3.
Two-year and median survival stratified by treatment and analytic methodology.
| Two-year survival (95% CI) | Median survival (95% CI) | |||
|---|---|---|---|---|
| Primary surgery | Primary chemotherapy | Primary surgery | Primary chemotherapy | |
| All Patients | ||||
| Observational | 0.56 (0.55-0.75) | 0.36 (0.34-0.38) | 28.8 (27.7-29.8) | 15.8 (15.1-17.1) |
| PS reweighted (matched) | 0.49 (0.46-0.51) | 0.40 (0.38-0.43) | 23.5 (21.6-24.6) | 18.5 (17.4-19.5) |
| PS reweighted (IPTW) | 0.54 (0.53-0.55) | 0.45 (0.44-0.47) | 27.2 (26.0-28.2) | 21.0 (20.1-21.5) |
| Instrumental variable | ||||
| <Median value of IV | 0.49 (0.48-0.51) | 23.8 (22.8-24.8) | ||
| ≥Median value of IV | 0.50 (0.48-0.52) | 24.1 (23.2-25.5) | ||
| Intended Treatment | ||||
| Observational | 0.64 (0.63-0.66) | 0.58 (0.55-0.62) | 34.7 (33.1-36.1) | 28.1 (26.2-30.5) |
| PS reweighted (matched) | 0.63 (0.60-0.67) | 0.59 (0.55-0.62) | 32.7 (30.4-35.6) | 28.2 (26.2-30.8) |
| PS reweighted (IPTW) | 0.64 (0.63-0.66) | 0.58 (0.56-0.59) | 34.7 (33.1-36.1) | 28.1 (27.1-28.8) |
| Instrumental variable | ||||
| <Median value of IV | 0.62 (0.61-0.64) | 32.5 (31.3-34.8) | ||
| ≥Median value of IV | 0.64 (0.62-0.66) | 35.9 (33.5-37.6) | ||
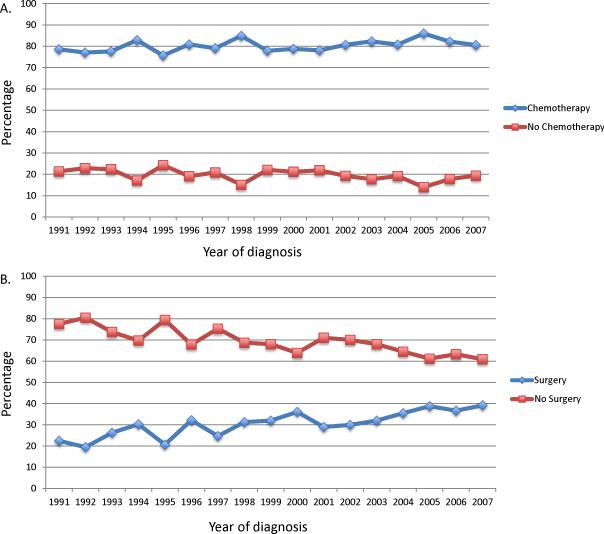
Among patients in the primary surgery group, 80.2% went on to receive postoperative chemotherapy while 31.9% of patients who began treatment with chemotherapy ultimately underwent surgery (Figure 2) (Supplemental table 4). Given that a substantial number of elderly women with ovarian cancer fail to complete both surgery and chemotherapy, regardless of which is the initial treatment, we performed a second analysis of intended treatment, i.e., those patients who received both surgery and chemotherapy. Although the influence on survival was more modest, the observational analysis, propensity score matched and propensity score IPTW analyses all suggested that survival was superior for women treated with primary surgery. In contrast, in the IV analysis the hazard ratios for both cancer-specific (HR=0.44; 95% CI, 0.24-0.84) and overall (HR=0.57; 95% CI, 0.32-1.01) survival favored a strategy of primary chemotherapy.
Figure 2.
Initiation of intended treatment (chemotherapy after primary surgery in the primary surgery group or surgery after primary chemotherapy in the primary chemotherapy group) in women stratified by initial treatment (primary surgery or neoadjuvant chemotherapy). A. Receipt of chemotherapy after primary surgery. B. Receipt of surgery after primary chemotherapy.
Discussion
While there is strong selection bias to treat women with the worst prognosis with neoadjuvant chemotherapy, our findings suggest that there is no evidence that survival for primary surgery and neoadjuvant chemotherapy differ significantly, especially in the subset of patients that received combination treatment. Treatment outcomes among elderly women in the U.S. are comparable to a recent randomized trial comparing neoadjuvant chemotherapy to primary cytoreduction.3
Numerous studies have examined the feasibility and outcome of neoadjuvant chemotherapy for advanced stage ovarian cancer.4-8,27-29 The majority of these investigations have been single institution, descriptive studies or retrospective comparisons. Many of these studies have noted decreased morbidity and an increased rate of optimal cytoreduction with a strategy of neoadjuvant chemotherapy.4,7,27 Despite the potential benefits of neoadjuvant chemotherapy, a 2007 meta analysis of 26 studies concluded that survival with primary chemotherapy was inferior to upfront cytoreduction.6 A major concern for interpreting data from these observational studies stems from the strong selection bias that influences non-randomized treatment choices. Women who receive neoadjuvant chemotherapy are more often older, have more comorbid conditions, and have tumors of higher grade and stage.4,8
Both propensity score and instrumental variable analysis have been proposed to help overcome the inherent selection bias and confounding of observational data.11-13
Instrumental variable analyses have the additional benefit of limiting the effects of unmeasured confounding factors.11-14,30-32 Hadley and colleagues compared conservative management and radical prostatectomy using multivariable regression, propensity score adjustment, and instrumental variable analysis. Similar to our findings, the investigators noted that although regression and propensity score adjustment favored the surgical intervention, the instrumental variable analysis found no difference in survival and was similar to data from a randomized trial.11,33
Our findings raise a number of concerns. First, a relatively large number of patients, irrespective of initial treatment, did not complete therapy with both chemotherapy and surgery. Nineteen percent of women never initiated chemotherapy after surgery while fewer than a third of women treated with neoadjuvant chemotherapy ultimately underwent surgery. It is unclear why so few women ultimately underwent surgery in our cohort. We hypothesize that some patients were receiving palliative rather than curative intent chemotherapy and alternatively, some women had disease progression while receiving chemotherapy. The inclusion of those patients treated palliatively in the primary chemotherapy cohort undoubtedly biased survival estimates in the primary analysis. Our findings that survival was more favorable in women treated with neoadjuvant chemotherapy in the stratified analysis of intended treatment are provocative and warrant further investigation.
The second concern raised by these data is the poor survival we noted for both treatment strategies. One criticism of the EORTC's randomized trial for advanced-stage ovarian cancer was the poor survival as a whole (30 months for neoadjuvant chemotherapy and 29 months for primary surgery).3,9,10 When compared to selected institutional series and cooperative group trials these survival results are clearly inferior.34-37 However, the survival estimates we noted from our population-based analysis of elderly women are also clearly more modest and in line with the EORTC data; in the unadjusted cohort median survival among women who underwent primary surgery was 29 months. These findings highlight the difficulty in generalizing results from randomized controlled trials to real world populations.38
We acknowledge a number of important limitations. Most importantly, we were unable to determine whether the intent of treatment was curative or palliative. Although the inclusion of women treated palliatively likely biased the survival estimates in the observational cohort, use of the IVA helps to mitigate this concern. Similarly, important prognostic factors for ovarian cancer including tumor distribution and amount of residual disease are lacking but should have been accounted for in the IV. While we attempted to capture patients who underwent some of treatment directed at improving survival, we recognize that exclusion of patients who survived <6 months from diagnosis may have biased our estimates of survival. We recognize IVA depends on the validity of the exogenous variable. A relationship between hospital referral region and survival is unlikely, but not impossible. However, as noted above, region has been used as an acceptable instrument in several similar studies and in our analysis region satisfied the statistical tests for instrumental variable assumptions. While IVA corrects for unmeasured confounders, these factors remain unobserved and limit the possibility to identify factors that may better influence treatment decisions and policies.
Which patients should receive neoadjuvant chemotherapy for ovarian cancer? A survey of gynecologic oncologists in the U.S. found that most practitioners report use of neoadjuvant chemotherapy in fewer than 10% of cases while some institutional series would suggest that approximately 20-50% of women receive neoadjuvant chemotherapy.4,7,39 Some centers in Europe report that neoadjuvant chemotherapy is used in closer to half of their patients with advanced stage disease.40 Our findings suggest that a strategy of neoadjuvant chemotherapy may be a reasonable treatment option for elderly women with ovarian cancer. Particularly in women who undergo surgery after induction chemotherapy, survival with neoadjuvant chemotherapy did not differ significantly from primary cytoreduction.
Supplementary Material
Precis.
Use of neoadjuvant therapy has increased over time. Survival with neoadjuvant chemotherapy compares favorably with primary surgery in elderly women in the U.S.
Acknowledgements
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01CA134964) are recipients of grants from the National Cancer Institute. Dr. Tsui is the recipient of a fellowship from the National Cancer Institute (T32 CA09529).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Information Services, and the Office of Strategic Planning, HCFA; Information Management Services (IMS), Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
The authors have no conflicts of interest or disclosures.
References
- 1.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–4. [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 4.Everett EN, French AE, Stone RL, et al. Initial chemotherapy followed by surgical cytoreduction for the treatment of stage III/IV epithelial ovarian cancer. Am J Obstet Gynecol. 2006;195:568–74. doi: 10.1016/j.ajog.2006.03.075. discussion 74-6. [DOI] [PubMed] [Google Scholar]
- 5.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103:1070–6. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Bristow RE, Eisenhauer EL, Santillan A, Chi DS. Delaying the primary surgical effort for advanced ovarian cancer: a systematic review of neoadjuvant chemotherapy and interval cytoreduction. Gynecol Oncol. 2007;104:480–90. doi: 10.1016/j.ygyno.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol. 2007;105:211–7. doi: 10.1016/j.ygyno.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PE, Rutherford TJ, Chambers JT, Kohorn EI, Thiel RP. Neoadjuvant chemotherapy for advanced ovarian cancer: long-term survival. Gynecol Oncol. 1999;72:93–9. doi: 10.1006/gyno.1998.5236. [DOI] [PubMed] [Google Scholar]
- 9.Chi DS, Bristow RE, Armstrong DK, Karlan BY. Is the easier way ever the better way? J Clin Oncol. 2011;29:4073–5. doi: 10.1200/JCO.2011.35.9935. [DOI] [PubMed] [Google Scholar]
- 10.Chi DS, Musa F, Dao F, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT). Gynecol Oncol. 2012;124:10–4. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 11.Hadley J, Yabroff KR, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102:1780–93. doi: 10.1093/jnci/djq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297:278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YF, Montie JE, Shahinian VB. Reducing bias in the assessment of treatment effectiveness: androgen deprivation therapy for prostate cancer. Med Care. 2012;50:374–80. doi: 10.1097/MLR.0b013e318245a086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. Jama. 1994;272:859–66. [PubMed] [Google Scholar]
- 15.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–45. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 16.Sheets NC, Goldin GH, Meyer AM, et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. Jama. 2012;307:1611–20. doi: 10.1001/jama.2012.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JD, Neugut AI, Wilde ET, et al. Physician characteristics and variability of erythropoiesis-stimulating agent use among Medicare patients with cancer. J Clin Oncol. 2011;29:3408–18. doi: 10.1200/JCO.2010.34.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright JD, Herzog TJ, Neugut AI, et al. Effect of radical cytoreductive surgery on omission and delay of chemotherapy for advanced-stage ovarian cancer. Obstet Gynecol. 2012;120:871–81. doi: 10.1097/AOG.0b013e31826981de. [DOI] [PubMed] [Google Scholar]
- 19.Wright J, Doan T, McBride R, Jacobson J, Hershman D. Variability in chemotherapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197–203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Neoadjuvant chemotherapy in the Medicare cohort with advanced ovarian cancer. Gynecol Oncol. 2011 doi: 10.1016/j.ygyno.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011;122:100–6. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–9. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Sax FL, MacKenzie CR, Fields SD, Braham RL, Douglas RG., Jr. Assessing illness severity: does clinical judgment work? J Chronic Dis. 1986;39:439–52. doi: 10.1016/0021-9681(86)90111-6. [DOI] [PubMed] [Google Scholar]
- 24.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 25. [February 5, 2013];The Dartmouth Atlas of Health Care. (at http://www.dartmouthatlas.org.)
- 26.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. Journal of health economics. 2008;27:531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–20. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 28.Vergote I, Amant F, Kristensen G, Ehlen T, Reed NS, Casado A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. Eur J Cancer. 2011;47(Suppl 3):S88–92. doi: 10.1016/S0959-8049(11)70152-6. [DOI] [PubMed] [Google Scholar]
- 29.Inciura A, Simavicius A, Juozaityte E, et al. Comparison of adjuvant and neoadjuvant chemotherapy in the management of advanced ovarian cancer: a retrospective study of 574 patients. BMC Cancer. 2006;6:153. doi: 10.1186/1471-2407-6-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wisnivesky JP, Halm EA, Bonomi M, Smith C, Mhango G, Bagiella E. Postoperative radiotherapy for elderly patients with stage III lung cancer. Cancer. 2012;118:4478–85. doi: 10.1002/cncr.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks JM, Chrischilles EA, Landrum MB, et al. Survival implications associated with variation in mastectomy rates for early-staged breast cancer. International journal of surgical oncology. 2012;2012:127854. doi: 10.1155/2012/127854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob BJ, Moineddin R, Sutradhar R, Baxter NN, Urbach DR. Effect of colonoscopy on colorectal cancer incidence and mortality: an instrumental variable analysis. Gastrointestinal endoscopy. 2012;76:355–64. e1. doi: 10.1016/j.gie.2012.03.247. [DOI] [PubMed] [Google Scholar]
- 33.Hadley J, Barrett MJ, Penson DF, Saigal CS, Potosky AL. Response. JNCI. 2011;103:1134–35. [Google Scholar]
- 34.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–64. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 35.Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 37.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong K. Methods in comparative effectiveness research. J Clin Oncol. 2012;30:4208–14. doi: 10.1200/JCO.2012.42.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dewdney SB, Rimel BJ, Reinhart AJ, et al. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119:18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Vergote I, Leunen K, Amant F. Primary surgery or neoadjuvant chemotherapy in ovarian cancer: what is the value of comparing apples with oranges? Gynecol Oncol. 2012;124:1–2. doi: 10.1016/j.ygyno.2011.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.