Abstract
Developing novel fish gelatin films with better mechanical properties than mammalian gelatin is a challenging but promising endeavor. Studies were undertaken to produce fish gelatin films by combining treatments with different sugars (ribose and lactose) followed ‘by’ ‘and’ ultraviolet (UV) radiation, as possible cross-linking agents. Increase in tensile strength and percent elongation at break was recorded, which was more significant in films without sugars that were exposed to UV radiation. Films with added ribose showed decreased solubility after UV treatment and exhibited higher swelling percentage than films with added lactose, which readily dissolved in water. FTIR spectra of all the films showed identical patterns, which indicated no major changes to have occurred in the functional groups as a result of interaction between gelatin, sugars and UV irradiation. The results of this study could be explored for commercial use, depending on industrial needs for either production of edible films or for food packaging purposes.
Keywords: Fish gelatin; Films, Ultraviolet radiation; Lactose; Ribose
Introduction
Food researchers are showing renewed interest in developing readily degradable, environmentally friendly, biopolymer films that could be used either to replace synthetic polymers in food packaging or as a nutritional supplement in the form of edible films (Karim and Bhat 2008, 2009). Attempts have been made to utilize natural polysaccharides, proteins, and lipid biopolymers to develop edible and non-edible packaging materials (Voon et al. 2011). Biopolymer films can improve food quality by efficiently acting as a barrier against moisture, gas, and aroma, thus offering protection for a food product after the primary package is opened. Generally, protein-based films is considered more beneficial in fulfilling nutrient supplementation and of late, additional research efforts have been focused on utilizing various types of naturally available protein sources (plant and animal origin). Among the various types of films investigated and developed, biopolymer films based on proteins have shown best mechanical properties. Some of the edible films have been prepared from dried soluble protein such as casein, serum albumin, and egg albumin (Guilbert 1986). Although protein-based films have been proclaimed to have wide applicability in the food industry, poor water vapor resistance and lower mechanical strength in comparison with synthetic polymers have limited their applications (Chambi and Grosso 2006).
Gelatin, a heterogeneous mixture of water-soluble proteins (of high average molecular weight) is derived by hydrolytic action from collagen. Recently, gelatin has gained high attention in food industries due to its unique properties (Saha and Bhattacharya 2010). Gelatin films are also being explored due to their easy availability and biodegradable nature (Karim and Bhat 2008, 2009). However, edible or packaging films produced from gelatin do not posses the desired mechanical and water vapor barrier properties, which limit their commercial use. To overcome this problem, researchers have tested physical (e.g., radiation treatments, ultrasound) and chemical (e.g., aldehydes, especially glutaraldehyde, calcium salts) treatments along with the use of natural plant products like phenolic compounds (e.g., tannic acid, ferrulic acid) for their ability to improve the cross-linking properties of films (Chambi and Grosso 2006). Previously, Zhang and Han (2006) reported the development of edible films using monosaccharides as plasticizers (mannose, glucose, fructose). Some sugars, especially ribose, induce Maillard cross-linking after mild heat treatments and are able to cross-link proteins (Hellsten et al. 2004). To a certain extent, lactose also has been reported to induce cross-linking (Venkatachalam et al. 1993).
In the present study, we used fish gelatin as the raw material for preparation of films. In recent years fish gelatin has gained importance, mainly because of religious sentiments (e.g., Judaism and Islam forbid the use of pig gelatin, whereas Hindus do not use gelatin produced from cows), the low production cost, and its easy availability worldwide. Fish gelatin has also been considered to be a better alternative than mammalian gelatin because of safety aspects (e.g., bovine spongiform encephalopathy from cow products) (Karim and Bhat 2008, 2009).
Ultraviolet (UV) radiation technology is one of the most preferred physical modes of decontamination employed in food industries. This technology possesses several advantageous features such as: ease of handling, cost effectiveness, and low penetration power compared to other sources of ionizing radiation (e.g., gamma, electron beam, or X-rays). UV treatment is reported to offer better cross-linking of collagen and gelatin (Wallner-Pendleton et al. 1994), and are capable of readily polymerizing a range of monomers. UV-initiated polymerization is generally accomplished by indirect bond cleavage. Being a weaker form of electromagnetic radiation than that of ionizing radiation, UV radiation has great potential for modifying physical and mechanical properties of protein-based films.
To our knowledge, no previous studies have characterized the mechanical properties of fish gelatin films developed using sugars or UV irradiation. Thus, the main objective of this study was to investigate the changes induced in fish gelatin films (via possible improvement in cross-linking) by the addition of the sugars such as: ribose (an aldo-pentose monosaccharide having five carbon atoms and an aldehyde functional group in a linear form) and lactose (a disaccharide composed of β-D- lactose and β-D-glucose molecules which are bonded via β1-4 glycosidic linkage) combined with UV irradiation as cross-linking agents. As both UV and sugars can impart cross-linking effects, they were used in combination. We also evaluated some of the functional properties of the films to provide baseline data for their future application in food industries.
Material and methods
Materials, preparation of films, and UV irradiation
Fish gelatin samples (in the form of granules) were procured from Croda Colloids Ltd. (Market Harborough, UK). The moisture content of fish gelatin granules used in the present study was 10.93% (± 0.02) (as determined by oven drying at 105 ± 1 °C) and had a gel strength of 177.8 g. Fish gelatin films were prepared (in replicates of three) according to the casting method. In brief, the gelatin film-forming solutions were prepared by dissolving granules of fish gelatin into distilled water to obtain a concentration of 5 g/100 ml (60 °C for 1 h) followed by cooling at room temperature (25 ± 1 °C for 15 min). Sugars (ribose or lactose) were added into the gelatin solution to obtain a final concentration of 1 g/100 g and 2 g/100 g (w/w) gelatin granule, and the solutions then were mixed thoroughly using a magnetic stirrer upon addition of glycerol (1% w/w).
For UV irradiation, the prepared samples in solution form were spread uniformly on a sterile oval aluminum plates (diameter 15 × 15 cm) and then exposed to a UV light source (253.7 nm; 30 W; lamp type G40T10; Sankyo-Denki Co., Tokyo, Japan) in a laminar flow cabinet at a distance of 30 cm from the surface (Pro-Lab, Stratford Upon Avon UK) for a time period of 60 min. The wavelength of 253.7 nm is routinely used in UV irradiation studies (Wallner-Pendleton et al. 1994). The time period of exposure was based on results of one of our earlier studies (Bhat and Karim 2008). On an average, the solution received a UV radiation dose of 2.158 J/m2.
After exposure, the samples were transferred aseptically onto sterile polyacrylic casting plates (16 × 16 cm) followed by oven drying at 40 °C for 24 h for film formation. Film thickness was controlled by casting the same amount of film-forming solution (80 mL) on each plate. Conditioning of samples was performed prior to use, wherein the prepared films were kept in a desiccator maintained at 0% relative humidity (RH) (silica gel) followed by 52% RH (saturated magnesium nitrate) for 24 h at 30 °C. Gelatin without the addition of sugars and exposure to UV light served as the control.
FTIR analysis
The spectra of the films were recorded by Fourier transform infrared (FTIR) spectrometry (System 2000, Perkin Elmer Wellesly, MD, USA). The light source of transmittance was 650–4000 cm−1. Film strips (10 × 10 cm) were placed on zinc selenide plates and spectra were recorded as attenuated total reflectance. The spectra obtained were used to determine possible interactions of functional groups between films cross-linked with sugar and after UV treatments.
Mechanical properties
Tensile strength (TS) and elongation at break (EB) of the films were measured using a texture analyzer (Stable Micro System, TA/HD 100 model, Surrey, UK) based on the ASTM D-882 standard method (ASTM 1981). Film strips (10.0 × 2.0 cm) from each formulation were cut and clamped between the tensile grips. Film strips placed between pneumatic grips were stretched at a cross head speed of 5.0 mm/s, with an initial distance between the grips maintained at 100 mm. All the measurements were recorded at at 25 ± 1 °C. TS was calculated by dividing the maximum force at break by the cross-sectional area of the film (expressed in Mpa), while EB was calculated based on the length extended and the original length of the films. Measurements were made in replicates of five. Prior to the experiment the film strips were pre-conditioned at 25 ± 1 °C at 52% RH for 48 h.
Film thickness
Film thickness was measured using a micrometer (dial thickness gauge 7301; Mitutoyo Co., Tokyo, Japan) with a sensitivity of 0.01 mm. Overall, 10 thickness measurements were taken on each film and the average values were recorded.
Water vapor permeability
Water vapor permeability (WVP) of the films was measured according to the ASTM E96-95 (1995) with slight modification, as described by Pranoto et al. (2007). Individual films were sealed as a thin covering (patch) onto a glass permeation cell/cup (4.5 × 2.8 cm) containing distilled water (up to 10 mm); the film was tightly sealed so that it was air-tight over the cup mouth. The sealed cup was then placed in a desiccator in which the RH was maintained at 50% and placed in an incubator, with the temperature maintained at 30 °C. The weight of each cup was measured every 2 h upto 12 h under this controlled environment. Weight loss over time was measured, and the slope of moisture loss over time was determined using linear regression analysis. The water vapor transmission rate (WVTR) was calculated from the slope of the straight line divided by the test area, whereas WVP (g mm/m2 h kPa) was calculated as:
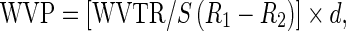 |
where S = saturation vapor pressure (Pa) of water at test temperature, R1 = RVP in the permeation cell, R2 = RVP in the desiccator, and d = film thickness (m) (RVP = relative vapor pressures).
Water solubility
We used the method described by Romero-Bestida et al. (2005) to determine the water solubility of the films. In brief, each of the prepared films was cut uniformly (2 × 3 cm) and the pieces were stored in a desiccator with silica gel maintained at 0% RH for 24 h. Samples were weighed to the nearest 0.0001 g and then were dispersed in beakers containing 80 mL of deionized water. The samples were maintained under constant agitation for 1 h at room temperature (approximately 25 ± 1 °C). The remaining pieces of film after soaking were filtered through filter paper (Whatman No.1), followed by oven drying (at 60 °C) until a constant weight was attained. Samples were measured in replicates of three, and the percentage of total soluble matter (% solubility) was calculated as follows:
 |
Swelling properties
Each film was cut into 2.5–2.5 cm pieces and weighed in air-dried conditions (W1), followed by immersion in distilled water (25 ± 1 °C) for 2 min. Wet samples were wiped with blotting paper to remove excess water and weighted again (W2). The amount of adsorbed water was calculated as
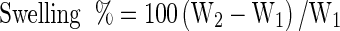 |
Where, W2 and W1 are the weights of the wet and the air-dried samples, respectively (Bigi et al. 2004). Measurements were taken in triplicates and the values were averaged.
Color measurement
The color of each of the films surface was measured using the Hunter Colorimeter (Spectrophotometer CM-3500 d, Minolta Co. Ltd, Osaka, Japan) and the Hunter color (L*-, a*-, and b*-) values were reported. Prior to the analysis, the instrument was calibrated to standard black and white tiles. A large size aperture was used and the measurement was conducted in triplicate through a computerized system by using Spectra Magic software (version 2.11, Minolta Cyberchrome Inc. Osaka, Japan).
Statistical analysis
Data from this study (samples run in triplicate) are represented as mean values ± standard deviation (SD). Analysis of variance (one way-ANOVA) was performed, and significant differences between mean values were determined by Tukey’s pair-wise comparison test at a level of significance of p < 0.05. Statistical analyses were conducted using SPSS 12.01 software (SPSS Inc., Chicago, IL, USA).
Results and discussion
FTIR analysis
FTIR spectroscopy has been successfully employed to interpret the conformational and structural changes undergone by proteins. Figure 1 shows the infrared spectra obtained for the fish gelatin films examined in this study. The spectra of all of the films showed identical patterns, which indicates that no major changes occurred in the functional groups of the gelatins as a result of interactions between gelatin, sugars, and UV irradiation. Fish gelatin films showed absorption bands around 1635 cm−1 (amide I, CO, and CN stretching), which is considered to be the most useful peak for infrared analysis of the secondary structure of protein like that found in gelatin (Muyonga et al. 2004). Another two absorption bands were seen at around 1529 cm−1 (amide II) and 1241 cm−1 (amide III). A prominent band was also observed at around 3291 cm−1, which might be attributed to NH stretching. Although a slight shift was observed in some of these bands after UV irradiation, it was not prominent. It should be noted that exposure of natural polymers to radiation not only results in better cross-linking but also might degrade the polymers (Kume et al. 2002). However, results from our study did not indicate any degradation to have occurred after UV treatments, as evidenced by the lack of new functional groups in the spectra.
Fig. 1.
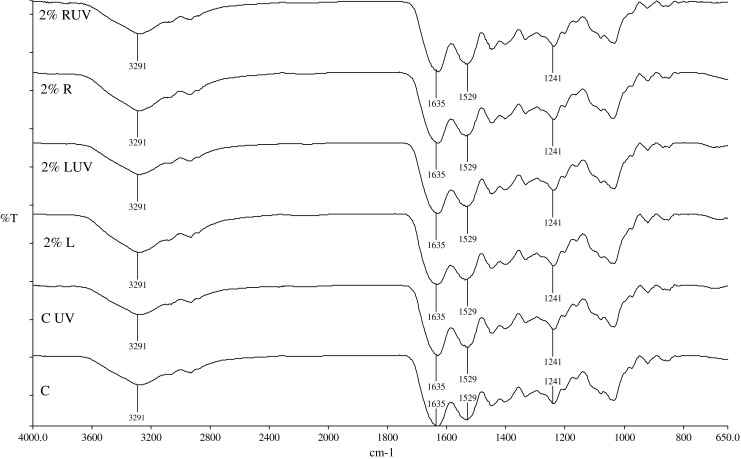
FTIR spectra of fish gelatin films; C, native fish gelatin film (control); CUV, control sample exposed to UV; 2%L, fish gelatin added with Lactose 2 g/100 g; 2%LUV, fish gelatin added with 2 g/100 g lactose and exposed to UV; 2% R fish gelatin added with Ribose 2 g/100 g; 2% R UV fish gelatin film added with Ribose 2 g/100 g and exposed to UV
Mechanical properties
Visual examination revealed that the films had a good appearance, homogenous texture, and were easy to handle at room temperature (25 ± 1 °C). Unlike the pure fish gelatin films, films produced by the incorporation of sugars had a pleasant aroma, which might be advantageous when used for edible packaging purposes.
The thickness, changes in TS, and EB of fish gelatin films with added sugars and exposed to UV irradiation are shown in Table 1. TS plays an important role in determining the mechanical properties of edible or packaging films developed for use in many food applications. TS are an indication of film strength, whereas E is an indicator of stretchability of films prior to breakage. A significant increase in the TS of the fish gelatin films after their exposure to UV radiation was recorded (Table 1). However, in general, higher TS values were recorded in films that did not contain the sugars. For the sugars-added films, the films with added ribose had higher TS than those with added lactose. A possible reason for this difference might be that ribose is a good cross-linking agent and might have induced Maillard cross-linking after initial heat treatments. However, the marked increase in TS might also be due to enhanced protein-protein interactions via non-covalent forces (e.g., Van de Walls, electrostatic, hydrogen bond, hydrophobic, etc.).
Table 1.
Effect of the addition of ribose and lactose sugars combined with UV irradiation on thickness, tensile strength, and elongation at break of fish gelatin films v
| Sample | Thickness in mm | Tensile strength as MPa | Percent Elongation |
|---|---|---|---|
| FG (control) w | 0.12 ± 0.050 a | 2.8 ± 0.45 a | 43.3 ± 1.74 e |
| FG + UV x | 0.13 ± 0.050 a | 12.0 ± 3.14 d | 1.3 ± 0.44 a |
| FG 1% L y | 0.12 ± 0.030 a | 1.9 ± 0.20 a | 34.4 ± 2.32 d |
| FG 1% L UV | 0.21 ± 0.100 c | 2.8 ± 0.90 a | 71.0 ± 9.60 h |
| FG 2% L | 0.13 ± 0.010 a | 2.8 ± 1.60a | 47.6 ± 5.50 f |
| FG 2% L UV | 0.15 ± 0.030 b | 3.9 ± 0.60 b | 64.1 ± 2.51 h |
| FG 1% R z | 0.11 ± 0.020 a | 4.0 ± 0.80 b | 40.1 ± 15.10 e |
| FG 1% R UV | 0.12 ± 0.010a | 4.7 ± 0.40 b | 51.9 ± 4.85 g |
| FG 2% R | 0.10 ± 0.010 a | 4.9 ± 0.30 b | 21.5 ± 14.13 c |
| FG 2% R UV | 0.12 ± 0.010 a | 9.4 ± 1.30 c | 15.5 ± 3.00 b |
vValues are the mean of three independent replicates ± standard deviation (SD); values with different letters in the same column were significantly different (p < 0.05) from each other
w FG Fish gelatin; x UV Ultraviolet radiation; y L Lactose; z R Ribose
Fish gelatin film containing 2% ribose and exposed to UV showed the second highest TS (the gelatin films exposed to UV without addition of sugars had the highest TS). Previous studies reported that the TS of films to be enhanced upon application of radiation treatments such as gamma, electron beams and UV (Sabato et al. 2007). The addition of more cross-linking agents might decrease the TS, as observed in our study; the addition of sugars followed by UV irradiation resulted in decreased TS as compared to pure gelatin films exposed to UV irradiation. Because the sugars were added to the solution before the gelatin films were exposed to UV, it is possible that some cross-linking might have already occurred via some of the functional groups, which in turn might make them unavailable for UV-induced modification/cross-linking. If this was the case, two of the following possible mechanisms can be inferred: either it is sugar induced cross-linking or UV-induced polypeptide aggregation (rather than actual cross-linking). Similar observations have been made by Taylor et al. (2005) on incorporation of higher amounts of cross-linking agents which tend to decrease the overall TS.
EB is a characteristic indicator of the flexibility of biopolymer films. A significant increase in EB (compared to control) was recorded in all of the samples except for the UV-treated gelatin films that lacked the addition of sugar (FG UV) and for the 2% ribose-incorporated films treated with UV irradiation (FG 2% UV) (see Table 1). This result contradicts the fact that percent elongation should decrease with an increase in tensile strength. Earlier, Lee et al. (2004) have opined that the strength and flexibility of a carbohydrate or protein composite film to be negatively correlated to each other. Sugars such as ribose or lactose, being macromolecules, possess lengthy chains of monomers and hence, apart from cross-linking reactions, they might enhance the macromolecular relaxations resulting in increased EB. Increase in the EB of films upon UV irradiation indicates the possibilities of better cross-linking, which might prove to be beneficial over other commonly employed chemical cross-linking agents.
Zhang and Han (2006) reported that fructose-, glucose-, and mannose-plasticized films had better or equal TS values than glycerol-, sorbitol, and maltitol-plasticized films, which indicates that monosaccharides are good plasticizers, and are comparable to commonly used polyols in the same molar ratio concentration. Higher TS of ribose-incorporated fish gelatin films over lactose-incorporated films might be attributed to the presence of an aldehyde functional group in the ribose sugar molecule. Several reports suggest that aldehyde-containing sugars offer better cross-linking (mainly due to Maillard reactions) when mixed with proteins (Gerrard et al. 2003).
The addition of small amounts of plasticizers such as glycerol into the film-forming solution can improve the processing feasibility and physical properties of the films. Rojas-Graü et al. (2008) have stated that incorporation of a good plasticizer like glycerol is a prerequisite for polysaccharide and protein-based edible films to enhance their flexibility and processability; the addition increases free volume or molecular mobility of polymers and thereby reduces the internal hydrogen bonding between polymer chains, which is accompanied by enhanced intermolecular spacing. The addition of glycerol into the fish gelatin films used in our study might be one of the major contributing factors to improved TS and EB.
Generally, radiation (via ionizing source) of aqueous protein solutions tends to generate hydroxyl radicals (.OH) (water radiolysis), which in turn are capable of generating unstable compounds. During UV irradiation, free radicals can be formed in proteins, and these free radicals might affect the amino acids, such as tyrosine and phenylalanine (aromatic amino acids), which are further involved in the formation of intermolecular covalent cross-linking bonds. Sarabia et al. (2000) reported that some of the amino acids, such as proline, hypdroxyproline, and alanine, in commercial fish gelatin sample to be responsible for the samples high visco-elastic properties.
From these observations, it is evident that the strength and flexibility of fish gelatin films could be modified and improved by application of UV irradiation and certain reducing sugars, such as ribose.
Water vapor permeability
The Water vapor permeability (WVP) of a biopolymer film is an important characteristic that can significantly influence the efficacy of its usage in food systems. WVP is also a measure wherein moisture can pass through a material very easily. Gelatin films, which are usually hydrophilic in nature, exhibit a positive slope relationship between thickness and WVP that results from variations in the water vapor (partial) pressure at the underside of the film during testing (McHugh et al. 1993). Hence, it is necessary to compare film thickness with WVP values. In the present study, there was no statistical difference recorded between film thicknesses among the different fish gelatin films tested (Table 1).
The WVP of the gelatin film decreased significantly with UV treatment (Table 2). Earlier studies on gluten, pectin, and soy-protein isolate films have exhibited similar trends, wherein the WVP decreased upon irradiation (Kang et al. 2005). In general, the presence of low molecular weight sugar might interrupt the interaction and can enhance hydrophilicity of films. Decrease in the WVP might be attributed to reduction in the chain-to-chain interactions that occur upon addition of a plasticizing agent, as reported earlier upon addition of sucrose (Veiga-Santos et al. 2005). Similarly, in this study, the WVP of sugar-incorporated films followed by UV treatments also showed a significant decrease compared to that of the control.
Table 2.
Water vapor permeability, water solubility, and swelling property of fish gelatin films with ribose and lactose sugar added and subjected to UV irradiation v
| Sample | WVP p (g mm/m2h kPa) | WS q (%) | SP r (%) |
|---|---|---|---|
| FG w (control) | 0.33 ± 0.090 d | 92.9 ± 5.75 f | 123.0 ± 7.23 b |
| FG + UV x | 0.29 ± 0.150 c | 93.3 ± 1.49 f | 132.6 ± 17.11 c |
| FG 1% L y | 0.20 ± 0.090 a | 87.0 ± 3.59 e | DS s |
| FG 1% L UV | 0.20 ± 0.020 a | 99.4 ± 0.08 h | DS |
| FG 2% L y | 0.29 ± 0.040 c | 78.3 ± 18.10 d | DS |
| FG 2% L UV | 0.25 ± 0.050 b | 98.1 ± 1.73 g | DS |
| FG 1% R z | 0.29 ± 0.020 c | 67.7 ± 10.40 c | 356.9 ± 15.90 e |
| FG 1% R UV | 0.24 ± 0.220 b | 36.9 ± 5.10 a | 104.6 ± 8.60 a |
| FG 2% R | 0.24 ± 0.040 b | 39.8 ± 7.48 a | 550.7 ± 5.50 e |
| FG 2% R UV | 0.26 ± 0.030 b | 41.9 ± 5.47 b | 216.9 ± 7.50 d |
vValues with different letters in the same column were significantly different (p < 0.05) from each other; values are the means of three replicates ± standard deviation (S.D.)
w FG Fish gelatin; x UV ultraviolet radiation; y L Lactose; z R Ribose
p WVP Water vapor permeability; q WS, water solubility; r SP, swelling properties
s DS dissolved
Water solubility and swelling properties
Water solubility is the measure of tolerance to water, and higher solubility of a film indicates lower water resistance (Handa et al. 1999). Table 2 shows the percentage solubility of the fish gelatin films with added sugars and exposed to UV irradiation.
Ribose-containing films showed significantly decreased solubility after UV treatment compared to lactose-containing films; this difference can be attributed to the possibility of higher cross-linking on incorporation of ribose. The presence of a high amount of cross-linkers in the film-forming solutions has been reported to lower the solubility (Ustunol and Mert 2004). Ealier, Galietta et al. (1998) reported decreased solubility of glycerol-plasticized films cross-linked with formaldehyde, and they suggested that the decrease was due to of the formation of covalent bonds. In the present study, the presence of possible multiple cross-linking agents (i.e., sugars and UV irradiation in the presence of glycerol) might have initiated the formation of covalent bonds, thereby leading to decreased water solubility of the ribose-incorporated and pure gelatin films.
The swelling property, which is related to the amount of water absorbed by films, is an important property of carbohydrate and protein films. Generally, biopolymer films produced from carbohydrates or proteins initially swell when they absorb water, which results in changes of their structure. Hence, details relevant to swelling characteristics are essential for successful application of biopolymer films. In this study, the swelling percentages (Table 2) indicated an insignificant increase in solubility after UV treatment of the control gelatin samples, while the opposite was true of sugar-incorporated films.
Ribose-incorporated films showed higher tolerance for water than lactose- incorporated films, which readily dissolved. The high solubility of lactose- incorporated films might be advantageous in some applications, such as edible films and candy wrap edible films, which need to dissolve quickly while they melt softly in the mouth.
Color
Table 3 lists the Hunter color values of the fish gelatin-based films incorporated with different concentrations of lactose and ribose sugar and exposed to UV irradiation. Irrespective of the samples, the Hunter color L* and a*- values showed a decreasing trend after UV treatment. In the lactose-incorporated films, the b* value decreased upon UV irradiation, whereas the opposite occurred in the ribose films. Ribose films showed more yellow color that might be attributed to the initiation of Maillard reaction. Changes in the L* value indicate a darker color, which was more prominent in the case of ribose-incorporated films. These results indicate that when irradiation is applied to obtain a better mechanical property, the transparency of the films would be affected. This finding agrees with results of the earlier work on the irradiation of starch-, pectin-, and gelatin-based films reported by Kim et al. (2008).
Table 3.
Hunter color values of the UV-irradiated fish gelatin films supplemented with lactose and ribose sugars v
| Sample | L* | a* | b* |
|---|---|---|---|
| FG w (control) | 2.1 ± 0.38 a | –0.24 ± 0.160 a | –0.34 ± 0.130 a |
| FG + UV x | 1.9 ± 0.17a | –0.18 ± 0.010 a | –0.24 ± 0.120 a |
| FG 1% L y | 2.5 ± 0.04 b | –0.35 ± 0.010 a | –0.67 ± 0.020 b |
| FG 1% L UV | 2.2 ± 0.47 b | –0.33 ± 0.090 a | –0.33 ± 0.060 a |
| FG 2% L | 2.1 ± 0.50 a | –0.45 ± 0.020 b | –0.58 ± 0.020 b |
| FG 2% L UV | 2.1 ± 0.06 a | –0.36 ± 0.060 a | –0.26 ± 0.010 a |
| FG 1% R z | 3.6 ± 0.33 c | –1.67 ± 0.320 c | 1.0 ± 0.07 c |
| FG 1% R UV | 3.4 ± 0.20 c | –1.60 ± 0.100 c | 1.9 ± 0.41 d |
| FG 2% R | 3.3 ± 0.00 c | –1.87 ± 0.050 d | 1.7 ± 0.01 d |
| FG 2% R UV | 3.5 ± 0.56 c | –1.44 ± 0.350c | 1.9 ± 0.06 d |
v Values with different letters in the same column were significantly different (p < 0.05) from each other; values are the means of three replicates ± standard deviation (S.D.)
w FG Fish gelatin; x UV Ultraviolet radiation; y L Lactose; z R Ribose
Conclusions
UV irradiation is a physical mean of food processing that has proven to be a useful technique for improving some of the mechanical properties (e.g., TS, EB and WVP) of fish gelatin films. Incorporation of the sugars (such as ribose) showed some promising results, which could be commercially explored depending on industrial needs. Additionally, combination treatments of fish gelatin with other natural cross-linking agents, especially plant-based ones (like polyphenols) along with UV irradiation merits investigation. Results of the present work might further pave the way for using fish gelatin as an alternative source to replace mammalian gelatin, at least in some food applications.
References
- Standard test methods for tensile properties of thin plastic sheeting, method D882–80a. Philadelphia: American Society for Testing and Materials; 1981. [Google Scholar]
- Standard test method for water vapor transmission of materials. Designation E96-95. Annual book of ASTM standards. Philadelphia: American Society for Testing and Materials; 1995. [Google Scholar]
- Bhat R, Karim AA. Ultraviolet irradiation improves gel strength of fish gelatin. Food Chem. 2008;113:1160–1164. doi: 10.1016/j.foodchem.2008.08.039. [DOI] [Google Scholar]
- Bigi A, Panzavolta S, Rubini K. Relationship between triple helix content and mechanical properties of gelatin films. Biomaterials. 2004;25:5675–5680. doi: 10.1016/j.biomaterials.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Chambi H, Grosso C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Res Int. 2006;39:458–466. doi: 10.1016/j.foodres.2005.09.009. [DOI] [Google Scholar]
- Galietta G, Di Gioia L, Guilbert S, Cuq B. Mechanical and thermomechanical properties of films based on whey proteins as affected by plasticizer and cross-linking agents. J Dairy Sci. 1998;81:3123–3310. doi: 10.3168/jds.S0022-0302(98)75877-1. [DOI] [Google Scholar]
- Gerrard JA, Brown PK, Fayle SE. Maillard cross-linking of food proteins II: the reaction of glutaraldehyde, formaldehyde and glyceraldehyde with wheat proteins in vitro and in situ. Food Chem. 2003;80:35–43. doi: 10.1016/S0308-8146(02)00232-7. [DOI] [Google Scholar]
- Guilbert S. Technology and application of edible protective films. In: Mathlouthi M, editor. Food packaging and preservation. New York: Elsevier Science Publishing; 1986. [Google Scholar]
- Handa A, Gennadios A, Froning GW, Kuroda N, Hanna MA. Tensile, solubility, and electrophoretic properties of egg white films as affected by surface sulfhydryl groups. J Food Sci. 1999;64:82–85. doi: 10.1111/j.1365-2621.1999.tb09865.x. [DOI] [Google Scholar]
- Hellsten Y, Skadhauge L, Bangsbo J. Effect of ribose supplementation on resynthesis of adenine nucleotides after intermittent training in humans. Am J Physiol-Regul Int Comp Physiol. 2004;286:R182–R188. doi: 10.1152/ajpregu.00286.2003. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Jo C, Lee NY, Kwon JH, Byun MW. A combination of gamma irradiation and CaCl2 immersion for a pectin-based biodegradable film. Carbohyd Polym. 2005;60:547–551. doi: 10.1016/j.carbpol.2005.02.016. [DOI] [Google Scholar]
- Karim AA, Bhat R. Gelatin alternatives for the food industry: recent developments, challenges and prospects. Trends Food Sci Tech. 2008;19:644–656. doi: 10.1016/j.tifs.2008.08.001. [DOI] [Google Scholar]
- Karim AA, Bhat R. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids. 2009;23:563–576. doi: 10.1016/j.foodhyd.2008.07.002. [DOI] [Google Scholar]
- Kim JK, Jo C, Park HJ, Byun MW. Effect of gamma irradiation on the physicochemical properties of a starch-based film. Food Hydrocolloids. 2008;22:248–254. doi: 10.1016/j.foodhyd.2006.11.010. [DOI] [Google Scholar]
- Kume T, Nagasawa N, Yoshii F. Utilization of carbohydrates by radiation processing. Radiat Phys Chem. 2002;63:625–627. doi: 10.1016/S0969-806X(01)00558-8. [DOI] [Google Scholar]
- Lee KY, Shim J, Lee HG. Mechanical properties of gellan and gelatin composite films. Carbohyd Polym. 2004;56:251–254. doi: 10.1016/j.carbpol.2003.04.001. [DOI] [Google Scholar]
- McHugh TH, Avena-Bustillos RJ, Krochta JM. Hydrophilic edible films: modified procedure for water vapor permeability and explanation of related thickness effects. J Food Sci. 1993;58:899–903. doi: 10.1111/j.1365-2621.1993.tb09387.x. [DOI] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Fourier transform infrared (FTIR) spectroscopy study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus) Food Chem. 2004;86:325–332. doi: 10.1016/j.foodchem.2003.09.038. [DOI] [Google Scholar]
- Pranoto Y, Lee CM, Park HJ. Characterizations of fish gelatin films added with gellan and k-carrageenan. LWT- Food Sci Technol. 2007;40:766–774. doi: 10.1016/j.lwt.2006.04.005. [DOI] [Google Scholar]
- Rojas-Graü MA, Tapia MS, Martín-Belloso O. Using polysaccharide-based edible coatings to maintain quality of fresh-cut Fuji apples. LWT-Food Sci Technol. 2008;41:139–147. doi: 10.1016/j.lwt.2007.01.009. [DOI] [Google Scholar]
- Romero-Bestida CA, Bello-Perez LA, Garcia MA, Martino MN, Solorza-Feria J, Zaritzky NE. Physicochemical and microstructural characterization of films prepared by thermal and cold gelatinization from non-conventional sources of starches. Carbohyd Polym. 2005;60:235–244. doi: 10.1016/j.carbpol.2005.01.004. [DOI] [Google Scholar]
- Sabato SF, Nakamurakare N, Sobral PJA. Mechanical and thermal properties of irradiated films based on Tilapia (Oreochromis niloticus) proteins. Radiat Phys Chem. 2007;76:1862–1865. doi: 10.1016/j.radphyschem.2007.02.096. [DOI] [Google Scholar]
- Saha D, Bhattacharya S. Hydrocolloids as thickening and gelling agents in food: a critical review. J Food Sci Technol. 2010;47:587–597. doi: 10.1007/s13197-010-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabia AI, Gómez-Guillén MC, Montero P. The effect of added salts on the viscoelastic properties of fish gelatin. Food Chem. 2000;70:71–76. doi: 10.1016/S0308-8146(00)00073-X. [DOI] [Google Scholar]
- Taylor MM, Marmer WN, Brown EM. Characterization of biopolymers prepared from gelatin and sodium caseinate for potential use in leather processing. J Am Leather Chem Assoc. 2005;100:149–159. [Google Scholar]
- Ustunol Z, Mert B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J Food Sci. 2004;69:129–133. [Google Scholar]
- Veiga-Santos P, Oliveira LM, Cereda MP, Alves AJ, Sxamparini ARP. Mechanical properties, hydrophilicity and water activity of starch-gum films: Effect of additives and deacetylated xanthan gum. Food Hydrocolloids. 2005;19:341–349. doi: 10.1016/j.foodhyd.2004.07.006. [DOI] [Google Scholar]
- Venkatachalam N, McMahon DJ, Savello PA. Role of protein and lactose interactions in the age gelation of ultra-high temperature processed concentrated skim milk. J Dairy Sci. 1993;76:1882–1894. doi: 10.3168/jds.S0022-0302(93)77521-9. [DOI] [PubMed] [Google Scholar]
- Voon HC, Bhat R, Easa AM, Liong MT, Karim AA (2011). Effect of addition of halloysite nanoclay and SiO2 nanoparticles on barrier and mechanical properties of bovine gelatin films. Food Bioprocess Technol (in press, doi: 10.1007/s11947-010-0461)
- Wallner-Pendleton EA, Sumner SS, Froning GW, Stetson LE. The use of ultraviolet radiation to reduce Salmonella and Psychrotrophic bacterial contamination on poultry carcasses. Poultry Sci. 1994;73:1327–1333. doi: 10.3382/ps.0731327. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Han JH. Plasticization of pea starch films with monosaccharides and polyols. J Food Sci. 2006;71:253–261. doi: 10.1111/j.1750-3841.2006.00075.x. [DOI] [Google Scholar]


