Abstract
In this research, bitter and sweet Lupin (Lupinus albus L.) seeds were used in bulgur production. The proximate chemical compositions and the contents of phytic acid, mineral, amino acid and fatty acid of raw material and processed lupin seeds as bulgur were determined. The sensory properties of bulgur samples were also researched. Bulgur process decreased ash, fat and phytic acid content of lupin seeds while significant increase (p < 0.05) was observed in protein content of bulgur compared with lupin seeds. Phytic acid losses in bitter and sweet lupin bulgurs were found as 18.8% and 21.3%, respectively. Generally sweet lupin seeds/bulgurs showed rich essential amino acids composition than that of bitter seeds/bulgurs. Linoleic and linolenic acid content of the lupin was negatively affected by bulgur process. Bitter lupin bulgur received lower scores in terms of taste, odor and overall acceptability than sweet lupin bulgur in sensory evaluation. Sweet lupin bulgur can be used as new legume-based product with high nutritional and sensorial properties.
Keywords: Lupin, Bulgur, Mineral, Phytic acid, Amino acid, Fatty acid
Introduction
Lupines are members of the family Leguminosea. The species Lupinus albus (white lupin), L. angustifolius (blue or narrow-leafed lupin) and L. luteus (yellow lupin) and L. mutabilis (Pearl lupin or Tarwi) have agricultural importance (Hondelmann 1984). Like other legume, lupin seeds are rich in protein, minerals and dietary fiber. Protein content of white lupin seed (33–47%) is higher than other legumes, and close to the soy protein content. Contrary to cereals, lupin proteins contain high amount of lysine and low amount of sulphur-containing amino acids (Dervas et al. 1999). The hull constitutes considerable part of the lupin seeds (20%) with a high content of dietary fibre and other valuable source of health promoting ingredients especially antioxidants (Gorecka et al. 2000). Lupin seeds with 34.44–39.42% dietary fiber content (3.64–5.21% soluble and 30.80–34.22% insoluble) may also be a potential source for the production of dietetic food (Martínez-Villaluenga et al. 2006). Lupin has considerable amount of oil (5–20%) in the whole seed although it is not oilseed crop (Mohamed and Rayas-Duarte 1995). Lupin seeds and flours are used in different cereal products as pasta, crisp, bread, cookie, cake and breakfast cereal (Dervas et al. 1999; Erbas et al. 2005).
Bulgur is produced from different cereals or legumes by cleaning, soaking, parboiling, drying, grinding and classification. It is semi-ready-to-eat food product with long shelf life. Wheat bulgur is an important food material for Turkish, Arabic, Mediterranean, North African and East European peoples because of its economical and nutritional values. In Turkey, durum wheat is preferred for bulgur production (Bayram et al. 2004). In different region of the world and also Turkey, oats (Baysal 1996), corn (Elgün et al. 1990), triticale (Singh and Dodda 1979), barley (Köksel et al. 1999), soybean (Bayram et al. 2004), common bean and chickpea (Bilgiçli 2009) are also used for bulgur production instead of durum wheat.
In our previous study, bulgur process was applied successfully on lupin seed in order to improve a new legume-based product. Sweet lupin seed showed better technological properties in bulgur process compared with bitter seed in terms of its lower soaking and cooking loss, higher volume increase and lighter bulgur color (Yorgancılar and Bilgiçli 2010). The objective of this research is to investigate the chemical changes of lupin seed during bulgur process that contains soaking, cooking, drying and grinding.
Material and methods
Material
In this study bitter lupin seeds and sweet lupin seeds were used. Bitter lupin seed is Turkish local population L. albus cv. (genotypes of this species is cultivated in Turkey, Doğanhisar). Sweet lupin seed (L. albus cv. Lutop) is originated France and kindly provided by Institute National de la Recherche Agranomique, France.
Bulgur production
Bulgur samples were prepared according to method given by Bilgiçli (2009). Lupin seeds soaked in distilled water for 12 h at room temperature. Then seeds cooked in water (1:1.5 w/v) at 90 °C for 1 h. For drying, cooked legumes were transferred to stainless-steel trays and dried in the oven at 80 °C until legume grains reach the moisture content of 10%. Legume samples were ground in a disk mill, and lupin bulgur was obtained over a 0.5-mm sieve.
Chemical properties
AACC methods were followed for determinations of moisture, ash, protein (Kjeldahl method using a conversion factor of 6.25) and fat content of the samples (AACC 1990).
Phytate phosphor and phytic acid content of the samples was measured by a colorimetric method according to Haugh and Lantzsch (1983). Phytic acid in the sample was extracted using a solution of HCl (0.2 N) and precipitated by solution of Fe III ammonium iron (III) sulphate 12H2O).
For analyzing the mineral contents of the seeds, approximately 0.5 g of ground seeds were put into a burning cup and mixed with 15 ml pure HNO3. The samples were incinerated in a microwave oven (MARS-5™ Cem Corp., Matthews, NC, USA) at 200 °C and dissolved ash was diluted to a certain volume with water. Concentrations were determined with an inductively coupled plasma atomic-emission spectrometer (ICP-AES) (Varian Vista Model, Australia) (Bubert and Hagenah 1987). The instrument was operated with a radiofrequency power of 0.7–1.5 kW (1.2–1.3 kW for axial); plasma gas flow rate (Ar) of 10.5–15 L/min(radial),15 L/min (axial); auxiliary gas flow rate (Ar) of 1.5 L/min; viewing height of 5–12 mm; copy and reading time of 1–5 s (maximum of 60 s); and copy time of 3 s(maximum of 100 s).
In order to determine the fatty acid composition, the fatty acids were converted to fatty acid methyl esters before analysis by shaking a solution of 0.2-g oil and 3 mL of hexane with 0.4 mL of 2-N methanolic potassium hydroxide. A Shimadzu (Kyoto, Japan) gas chromatograph, equipped with a flame ionization detector and a split/splitless injector, was used. Separations were made using a Teknokroma TR-CN100 (Barcelona, Spain) fused-silica capillary column (60 m · 0.25 mm i.d. · 0.20 μm film thickness). The carrier gas was nitrogen, with a flow rate of 1 mL/min. The temperatures of the injector, the detector and the oven were held at 220, 250 and 210 °C, respectively. The injection volume was 1 mL. Peaks were identified by comparison of their retention times with those of authentic reference compounds (Sigma–Aldrich, St. Louis, MO, USA). Fatty acid metal esters were determined by GC according to the method described by Slover and Lanza (1979) with minor modifications. Fatty acid metil esters were prepared using boron trifluoride in methanol (20% of BF3 in methanol) and extracted with n-hexane and then analyzed by GC.
Amino acid analyzes were performed according to Fleming and Terrell (1992) and Henderson et al. (2000). Chromatographic analysis of amino acids was conducted by the following experimental conditions: Instrument; Agilent 1100 HPLC, vacuum Degasser C.1379A, binary pump C.1312A, auto sampler C.1329A, column oven C.1316 A, fluorescence detector C.1316A, UV detector G 1314 A. HPLC columns; ZORBAX Eclipse-AAA, 4.6 × 150 mm, 3.5 μm. Mobile phase; A:0,4 mol/L NaH2PO4; B:45:45:10 MeOH : CAN : water, Mobile phase : 2 mL/min. Detector; Fluorescence detector, Ex: 340 Em :450 nm; UV detector :338 nm, Column temperature; 40° C, Stock standard; SIGMA (AAS18) amino acid standard.
Color measurement
Color of the samples was evaluated by measuring the L (100 = white; 0 = black), a (+, red; −, green) and b (+, yellow; −, blue) values using a Hunter Lab Color QUEST II Minolta CR-400 (Minolta Camera, Co., Ltd., Osaka, Japan) with illuminate D63 as reference. Values are the mean of three determinations. For color measurement, bulgur samples were ground in a blender (Moulinex Super Junior S, Paris, France), sieved from 500 mm opening screen and color measurement was made on these granulated material.
Sensory properties
Sensory properties of bulgur samples were determined according to Bilgiçli (2009). Bulgur samples (1,000 g) were boiled in water (2,000 mL) at 100 ± 5 °C. After all the boiling water was absorbed, a 100-g bulgur sample was served to the panelists at approximately 40 °C. Taste, odor, color, hardness, mouthfeel and overall acceptability values of the bulgur samples were rated on a 1–5 scale: 1—dislike extremely; 3—acceptable; and 5—like extremely.
Statistical analysis
The means, which were statistically different from each other, were compared using Duncan’s multiple comparison tests at 5% confidence interval. The TARIST (version 4.0, Izmir, Turkey) software was used to perform the statistical analyses. The experiments were carried out in duplicate and the analysis was performed in triplicate.
Results and discussion
Proximate chemical compositions of raw and processed lupin seeds are given in Table 1. Compared to raw material, ash and fat content decreased at 28.6% and 12.5% ratio in bitter lupin bulgur, and 12.5% and 15.5% in sweet one. In contrast ash and fat content, significant increase (p < 0.05) was observed in protein content of bitter and sweet bulgur. These changes could be resulted from removing lupin hull during bulgur production. Özboy and Köksel (1998) reported no change in protein content, but a slight decrease in ash content during wheat bulgur production.
Table 1.
Some chemical properties and color values of lupin seeds and bulgursa
| Properties | Raw material | Bulgur | ||
|---|---|---|---|---|
| Bitter | Sweet | Bitter | Sweet | |
| Moisture (%) | 9.1 ± 0.14 b | 8.7 ± 0.07 c | 11.4 ± 0.14 a | 11.8 ± 0.07 ba |
| Ashb (%) | 2.8 ± 0.03 c | 4.3 ± 0.04 a | 2.0 ± 0.03 d | 3.6 ± 0.03 b |
| Proteinb,c (%) | 33.0 ± 0.14 d | 36.0 ± 0.08 b | 35.5 ± 0.07 c | 38.1 ± 0.07 a |
| Fatb (%) | 12.0 ± 0.03 a | 9.7 ± 0.06 c | 10.5 ± 0.06 b | 8.2 ± 0.08 d |
| Phytic acidb (mg/100 g) | 848.0 ± 1.41 c | 1335.1 ± 1.13 a | 689.0 ± 1.70 d | 1050.4 ± 1.41 b |
| Phytate phosphorusb (mg/100 g) | 239.1 ± 0.57 c | 376.5 ± 0.71 a | 194.3 ± 0.71 d | 296.1 ± 0.84 b |
| Color values | ||||
| L* | 65.2 ± 0.03 d | 67.1 ± 0.07 b | 66.1 ± 0.06 c | 68.3 ± 0.11 a |
| a* | 7.0 ± 0.11 a | 3.4 ± 0.14 c | 6.8 ± 0.07 a | 5.5 ± 0.13 b |
| b* | 20.3 ± 0.11 c | 16.5 ± 0.11 d | 29.2 ± 0.08 b | 30.7 ± 0.12 a |
aDuncan’s multiple range test. Means (± standard error) with same letter within row are not significantly different (p < 0.05). Values are the average of triplicate measurements on the duplicate sample
bBased on dry matter
cN × 6.25
Phytic acid binds minerals and proteins and alters their solubility, functionality, digestibility and absorption (Rickard and Thompson 1997). In present study, bulgur process decreased the phytic acid contents of bitter and sweet lupin seeds at 18.8% and 21.3% ratio, respectively. In our previous study, reduction ratio of phytic acid in bulgur compared to raw seed were found between 25.2 and 32.0% for common bean bulgur and between 31.2 and 39.5% for chick pea bulgur. Bulgur production steps, cooking and dehulling are very effective for the destruction of phytic acid (Deshpande and Damodaran 1990). Vadivel and Biesalski (2011) reported decrease in bioactive compounds including phytic acid of ten different wild type legume grains after soaking and cooking processes.
According to De Boland et al. (1975), the differences in the loss of phytic acid contents during cooking could probably be explained on the basis that phytase activity at a temperature of 40–55 °C may degrade inositol hexaphosphate to the pentaphosphate or lower molecular weight. The soaking process before cooking may have increased the loss of phytic acid due to leaching of phytate ions into soaking water (Bishnoi et al. 1994).
Color values of raw material and bulgur samples are also given in Table 1. Lightness and yellowness values of the bitter and sweet bulgurs were higher than those of raw material. This increment in lightness and yellowness values at the end of bulgur process may be due to removing of hull. Redness value was not changed during bulgur process in bitter lupin seed. In contrast these findings, decrease in lightness and increase in yellowness values during the transition from legume grain (common bean or chickpea) to bulgur have been reported by Bilgiçli (2009). And also Güzel and Sayar (2012) reported significant (p < 0.05) decrease in lightness value of barlotto bean, chickpea, faba bean, and white kidney bean with different cooking method compared to raw material.
Mineral values of raw and processed lupin seeds are given in Table 2. While significant (p < 0.05) decrease was observed in potassium (K), magnesium (Mg), manganese (Mn) and zinc (Zn) contents of raw sweet and bitter lupin seed, phosphorus (P), calcium (Ca) and iron (Fe) contents were not changed significantly (p > 0.05) during bulgur production. Bilgiçli (2009) reported that, all minerals and trace elements were decreased in variable degrees at the end of the legume bulgur process compared to raw material, common bean and chickpea seeds. In the present study, the highest losses were observed in K and Mg among the investigated minerals. The amounts of these loses were 44.8 and 37.0% for bitter lupin bulgur and 38.5 and 26.1% for sweet lupin bulgur, respectively. These losses are due to leaching of minerals from the legume seeds into the water at different rates in soaking and cooking treatments, and removing lupin hull. Barampama and Simard (1995) reported that soaking and cooking processes caused considerable losses in soluble solids, especially vitamins and minerals.
Table 2.
Mineral values of lupin seeds and bulgursa,b
| Mineral (mg/100 g) | Raw material | Bulgur | ||
|---|---|---|---|---|
| Bitter | Sweet | Bitter | Sweet | |
| P | 350.0 ± 1.41 b | 410.4 ± 2.83 a | 346.7 ± 1.41 b | 408.0 ± 1.41 a |
| K | 760.1 ± 2.83 c | 1300.3 ± 2.83 a | 419.8 ± 2.13 d | 800.3 ± 2.12 b |
| Ca | 294.9 ± 2.70 a | 300.2 ± 2.71 a | 300.3 ± 2.85 a | 319.6 ± 2.13 a |
| Mg | 190.4 ± 0.14 a | 160.0 ± 0.28 b | 119.8 ± 0.28 c | 118.3 ± 0.17 d |
| Fe | 6.2 ± 0.01 a | 4.3 ± 0.04 b | 6.3 ± 0.01 a | 4.3 ± 0.06 b |
| Mn | 246.0 ± 0.71 c | 445.4 ± 1.27 a | 221.0 ± 1.13 d | 430.0 ± 0.71 b |
| Zn | 7.4 ± 0.03 b | 7.6 ± 0.03 a | 6.5 ± 0.06 c | 6.2 ± 0.03 d |
aDuncan’s multiple range test. Means (± standard error) with same letter within row are not significantly different (p < 0.05). Values are the average of triplicate measurements on the duplicate sample
bBased on dry matter
Legumes are very important mineral sources for human nutrition. Yorgancılar et al. (2009) reported mineral content of dehulled lupin and lupin hull as 561.2 and 153.2 mg/100 g for P, 23.02 and 32.38 mg/100 g for K, 379.2 and 1239.9 mg/100 g for Ca, 81.7 and 141.1 mg/100 g for Mg, 4.5 and 1.6 mg/100 g for Fe, 111.4 109.1 mg/100 g and for Mn and 6.0 and 2.4 mg/100 g for Zn, respectively.
The Recommended Dietary Allowances for adult males are 800 mg P, 1.6–2.0 g K, 800 mg Ca, 350 mg Mg, 10 mg Fe and 15 mg Zn. When 100 g (dry matter) of sweet lupin bulgur was consumed, 51.0%, 44.5%, 40.0%, 33.8%, 43.0% and 41.3% of Recommended Dietary Allowances for phosphorus, P, K, Ca, Mg, Fe and Zn respectively, can be provided in human body.
Essential amino acid composition of lupin seeds and bulgur samples are given in Table 3. All essential amino acids except methionine were increased between 13.51 and 125.0% for bitter lupin bulgur and between 8.3 and 57.1% for sweet one compared to their raw lupin seeds. These increments may be caused by removing of hull during bulgur production since protein and amino acid content of the hull is lower compared to the kernel.
Table 3.
Essential amino acid composition of lupin seeds and bulgursa,b
| Amino acid (g/100 g) | Raw material | Bulgur | ||
|---|---|---|---|---|
| Bitter | Sweet | Bitter | Sweet | |
| Arginine | 2.7 ± 0.05 d | 3.6 ± 0.05 b | 3.3 ± 0.04 c | 3.9 ± 0.05 a |
| Histidine | 0.74 ± 0.03 c | 0.84 ± 0.03 b | 0.84 ± 0.03 b | 0.98 ± 0.04 a |
| Isoleucine | 1.2 ± 0.04 c | 1.3 ± 0.03 b | 1.5 ± 0.01 a | 1.6 ± 0.03 a |
| Leucine | 2.3 ± 0.01 d | 2.7 ± 0.01 c | 2.8 ± 0.01 b | 3.1 ± 0.02 a |
| Lysine | 1.5 ± 0.04 d | 1.8 ± 0.03 c | 1.8 ± 0.04 b | 2.0 ± 0.02 a |
| Methionine | 0.08 ± 0.00 bc | 0.07 ± 0.01 c | 0.18 ± 0.02 a | 0.11 ± 0.02 b |
| Phenylalanine | 1.3 ± 0.04 c | 1.4 ± 0.04 b | 1.6 ± 0.04 a | 1.6 ± 0.05 a |
| Tryptophane | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a | 0.02 ± 0.00 a |
| Valine | 1.1 ± 0.02 c | 1.3 ± 0.04 b | 1.4 ± 0.03 a | 1.5 ± 0.02 a |
aDuncan’s multiple range test. Means (± standard error) with same letter within row are not significantly different (p < 0.05). Values are the average of triplicate measurements on the duplicate sample
bBased on dry matter
Fatty acid contents of the samples are presented in Table 4. Oleic acid was the dominant unsaturated fatty acid with 53.4–61.7% content in raw seeds and 53.9–63.1% content in bulgurs. Linoleic acid ranged between 18.9 and 25.6% in all investigated samples. Content of polyunsaturated fatty acid (linolenic acid) was decreased significantly (p < 0.05) at the end of the bulgur process. Erbaş et al. (2005) reported that oil of lupin seeds was composed of 13.5% saturated, 55.4% monounsaturated, and 31.1% polyunsaturated fatty acids, and oleic acid (55.4%) was the predominant fatty acid in lupin seed oil.
Table 4.
Fatty acid composition of lupin seeds and bulgursa,b
| Fatty acid (%) | Raw material | Bulgur | ||
|---|---|---|---|---|
| Bitter | Sweet | Bitter | Sweet | |
| Palmitic acid | 7.8 ± 0.06 c | 7.1 ± 0.02 d | 8.5 ± 0.04 a | 8.2 ± 0.08 b |
| Oleic acid | 61.7 ± 0.02 b | 53.4 ± 0.03 d | 63.1 ± 0.06 a | 53.9 ± 0.08 c |
| Linoleic acid | 18.9 ± 0.09 b | 25.6 ± 0.06 a | 18.1 ± 0.02 c | 25.3 ± 0.11 a |
| Linolenic acid | 8.0 ± 0.06 b | 8.6 ± 0.06 a | 6.5 ± 0.08 d | 7.5 ± 0.07 c |
| Stearic acid | 3.6 ± 0.06 b | 5.3 ± 0.05 a | 3.8 ± 0.09 b | 5.1 ± 0.07 a |
aDuncan’s multiple range test. Means (± standard error) with same letter within row are not significantly different (p < 0.05). Values are the average of triplicate measurements on the duplicate sample
bBased on dry matter
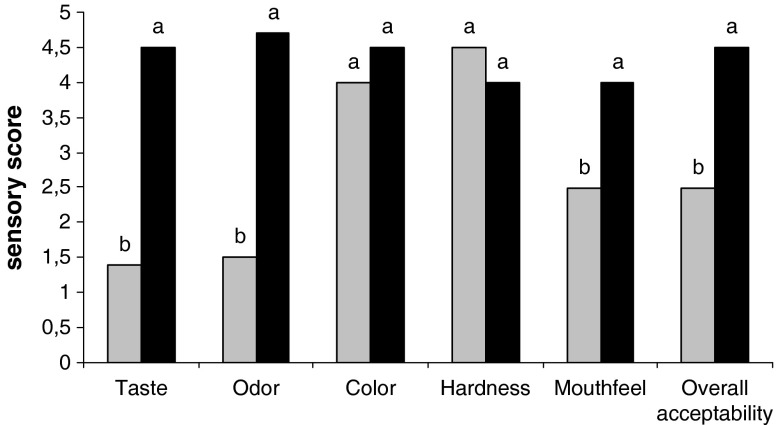
Sensory scores of bitter and sweet lupin bulgurs are shown in Fig. 1. While taste, odor and mouthfeel properties of the bitter bulgur had lower score than that of sweet one, color and hardness of the bulgurs were liked same degree by the panelists. Especially, taste and odor of the bulgurs directly affected overall acceptability scores. Usage of bitter lupin seeds in bulgur production decreased acceptability scores significantly (p < 0.05) due to bitter taste. 12 h soaking and cooking process can not removed all alkaloids which gives bitter taste.
Fig. 1.
Sensory properties of bitter (█) and sweet (█) bulgur samples (Same letter in the figure are not significantly different (p < 0.05). Values are the average of duplicate samples which evaluated by eight panelist)
Conclusion
In this study, the alternative usage of lupin seeds in bulgur production was implemented. The chemical and nutritional changes were also evaluated during bulgur production. Ash, fat, phytic acid K, Mg, Mn and Zn content of bulgur samples were found lower compared with their lupin seed. In contrast, bulgur processing caused significant increase (p < 0.05) in protein and amino acid content. A significant loss was observed in linoleic and linolenic acid content of bitter lupin bulgur compared to raw material. As conclusion, sweet lupin seed can be recommended for bulgur production with higher nutritional and sensorial properties except its higher phytic acid content compared to biter one.
Contributor Information
Mustafa Yorgancilar, Email: myorg@selcuk.edu.tr.
Nermin Bilgiçli, Phone: +90-332-2232937, FAX: +90-332-2410108, Email: nbilgicli@selcuk.edu.tr.
References
- Approved methods of the American association of cereal chemists. 8. St. Paul: AACC; 1990. [Google Scholar]
- Barampama Z, Simard RE. Effect of soaking, cooking and fermentation on composition, in vitro starch digestibility and nutritive value of common beans. Plant Food Hum Nutr. 1995;48:349–365. doi: 10.1007/BF01088494. [DOI] [PubMed] [Google Scholar]
- Bayram M, Öner MD, Kaya A. Influence of soaking on the dimensions and colour of soybean for bulgur production. J Food Eng. 2004;61:331–339. doi: 10.1016/S0260-8774(03)00137-7. [DOI] [Google Scholar]
- Baysal A (1996) Bulgur: Beslenme ve sağlik yönünden önemi. In: Proceedings of the Un-Bulgur Bisküvi Symposium, pp 23–31, Karaman, Turkey
- Bilgiçli N. Effects of cooking and drying processes on physical, chemical and sensory properties of legume based bulgur. J Food Proc Pres. 2009;3:590–604. doi: 10.1111/j.1745-4549.2008.00273.x. [DOI] [Google Scholar]
- Bishnoi S, Kheterpaul N, Yadav RK. Effect of domestic processing and cooking methods on phytic acid and polyphenol contents of pea cultivars. Plant Food Human Nutr. 1994;45:381–388. doi: 10.1007/BF01088088. [DOI] [PubMed] [Google Scholar]
- Bubert H, Hagenah WD. Detection and measurement. In: Boumans PWJM, editor. Inductively coupled plasma emission spectroscopy. New York: Wiley-Interscience; 1987. pp. 536–567. [Google Scholar]
- De Boland AR, Garner GB, O’dell BL. Identification and properties of phytate in cereal grains and oil seed products. J Agric Food Chem. 1975;23:1186–1190. doi: 10.1021/jf60202a038. [DOI] [PubMed] [Google Scholar]
- Dervas G, Doxastakis G, Zinoviadi S, Triandatafilikos N. Lupin flour addition to wheat flour doughs and effect on rheological properties. Food Chem. 1999;66:67–73. doi: 10.1016/S0308-8146(98)00234-9. [DOI] [Google Scholar]
- Deshpande SS, Damodaran S. Food legumes: chemistry and technology. In: Pomeranz Y, editor. Advances in cereal science and technology. St. Paul: AACC; 1990. pp. 147–226. [Google Scholar]
- Elgün A, Ertugay Z, Ceretel M. Corn bulgur: effects of corn maturation stage and cooking from on bulgur making parameters and physical and chemical properties of bulgur products. Cereal Chem. 1990;671:1–6. [Google Scholar]
- Erbas M, Certel M, Uslu MK. Some chemical properties of white lupin seeds (Lupinus albus L.) Food Chem. 2005;89:341–345. doi: 10.1016/j.foodchem.2004.02.040. [DOI] [Google Scholar]
- Fleming J, Terrell L (1992) Analysis of complex mixtures of amino acids using the HP 1050 modular HPLC, application note 228–212
- Gorecka D, Lambart SE, Janitz W, Sokolowska B. Composition of fractional and functional properties of dietary fiber of lupines (L. luteus and L. albus) Nahrung Foods. 2000;44(3):229–232. doi: 10.1002/1521-3803(20000701)44:4<229::AID-FOOD229>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Güzel D, Sayar S. Effect of cooking methods on selected physicochemical and nutritional properties of barlotto bean, chickpea, faba bean, and white kidney bean. J Food Sci Technol. 2012;49(1):89–95. doi: 10.1007/s13197-011-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugh W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereals product. J Sci Food Agric. 1983;34:1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Henderson JW, Ricker RD, Bidlingmeyer BA, Woodward C (2000) Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agilent Publication Number 5980-1193EN. Agilent Technologies, Palo Alto, CA
- Hondelmann W. The lupin-ancient and modern crop. Theo Appl Genet. 1984;68:1–8. doi: 10.1007/BF00252301. [DOI] [PubMed] [Google Scholar]
- Köksel H, Edney MJ, Zkaya B. Barley bulgur: effect of processing and cooking on chemical composition. J Cereal Sci. 1999;29:185–189. doi: 10.1006/jcrs.1998.0230. [DOI] [Google Scholar]
- Martínez-Villaluenga C, Frías J, Vidal-Valverde C. Functional lupin seeds (Lupinus albus L. and Lupinus luteus L.) after extraction of α-galactosides. Food Chem. 2006;98:291–299. doi: 10.1016/j.foodchem.2005.05.074. [DOI] [Google Scholar]
- Mohamed AA, Rayas-Duarte P. Composition of Lupinus albus. Cereal Chem. 1995;72:643–647. [Google Scholar]
- Özboy Ö, Köksel H. Bulgur üretiminin buğdaylarin bazi kimyasal özelliklerinde meydana getirdiği değisiklikler. Gida. 1998;23(6):449–457. [Google Scholar]
- Rickard ES, Thompson LU. Interactions and effects of phytic acid. In: Shahidi F, editor. Antinutrients and phytochemicals in foods. Washington, DC: American Chemical Society; 1997. pp. 294–312. [Google Scholar]
- Singh B, Dodda LM. Studies on the preparation and nutrient composition of bulgur from triticale. J Food Sci. 1979;44:449–452. doi: 10.1111/j.1365-2621.1979.tb03809.x. [DOI] [Google Scholar]
- Slover HT, Lanza E. Quantitative analysis of food fatty acids by capillary gas chromatography. J Am Oil Chem Soc. 1979;56:933–943. doi: 10.1007/BF02674138. [DOI] [Google Scholar]
- Vadivel V, Biesalski HK (2011) Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. J Food Sci Technol. doi:10.1007/s13197-010-0223-x [DOI] [PMC free article] [PubMed]
- Yorgancılar M, Atalay E, Babaoğlu M. Acılığı giderilmiş termiye tohumlarının mineral içeriği. Selçuk Tarım ve Gıda Bilimleri Dergisi. 2009;23(50):10–15. [Google Scholar]
- Yorgancılar M, Bilgiçli N. Utilazition of lupin seed in bulgur production. J Food Agric Environ. 2010;8(3–4):167–169. [Google Scholar]