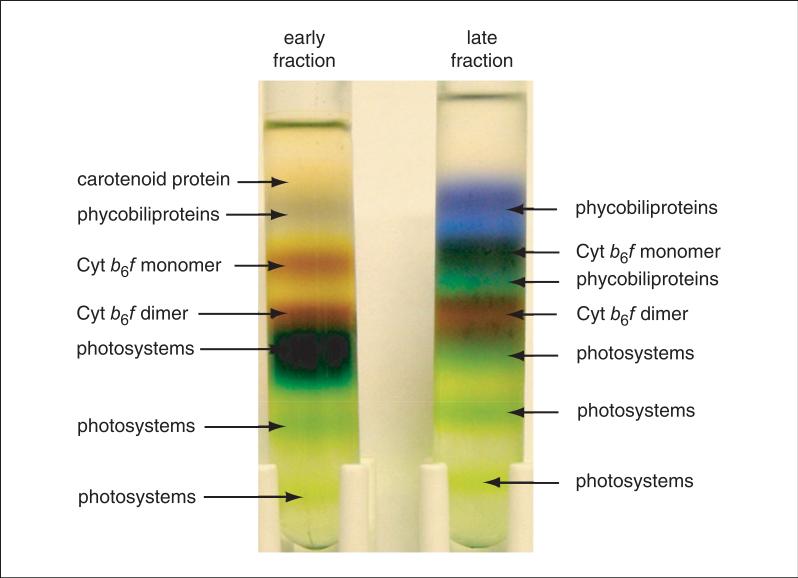
Figure 29.7.8.
Protein fractionation by sucrose density gradient centrifugation. Early protein fractions from the hydrophobic-interaction chromatography column are contaminated mainly with green photosystems, whereas late fractions have a significantly larger amount of phycobiliproteins. Dimeric Cyt b6f complex forms a brown band directly above the green photosystem band. A lower-density band of monomeric Cyt b6f complex is also observed. The dimeric Cyt b6f complex band is harvested into multiple fractions that are further purified by sucrose density gradient centrifugation to remove residual contaminants from the neighboring bands. The purified Cyt b6f is almost free of contaminants and is used for crystallographic studies. Figure originally published in the Journal of Biological Chemistry, Baniulis et al. (2009). For the color version, go to http://www.currentprotocols.com/protocol/ps2907.