Figure 2.
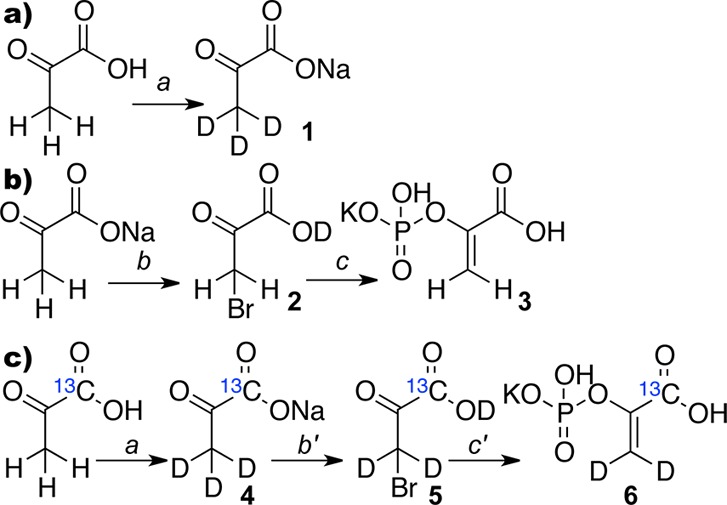
Step-wise optimization of deuterium exchange of pyruvic acid, synthesis of PEP from sodium pyruvate, and preparation of 1-13C-phosphoenolpyruvate-d2 (1-13C-PEP-d2, 6). (a) Deuterium exchange: (a) (i) D2O (450 mL), 100 °C, 5 h; (ii) 0.95 eq. NaHCO3; (iii) Rec. D2O/EtOH; sodium pyruvate-d3 (2, 54% yield, ratio C3D3O3– to C3D2HO3– = 1:0.28). (b) Potassium phosphoenolpyruvate synthesis based on sodium pyruvate: (b) 0.95 eq. H2SO4, 0.95 eq. Br2 (dry), CCl4; (c) (i) P(OMe)3, THF; (ii) H2O; (iv) KOH to pH ∼ 2.7; (v) Rec. H2O/EtOH; potassium phosphoenolpyruvate (3, 52% over two steps); (c) preparation for potassium 1-13C-phosphoenolpyruvate-d3 (1-13C-PEP-d3): (a) (i) D2O (450 mL), 100 °C, 5 h; (ii) 0.95 eq. NaHCO3; (iii) Rec. D2O/EtOH; sodium 1-13C-pyruvate-d3 (4, 75% yield, C213CD3O3– to C213CD2HO3– = 1:0.25). (b′) 0.95 eq. D2SO4, 0.95 eq. Br2 (dry), CCl4; (c′) (i) P(OMe)3, THF; (ii) D2O; (iii) KOH to pH ∼ 2.7; (iv) Rec. H2O/EtOH (6, 43% over 2 steps, C213CH2D2O6P– to C213CH3DO6P– to C213CH4O6P– = 1:0.10:0.05).
