Fig. 1.
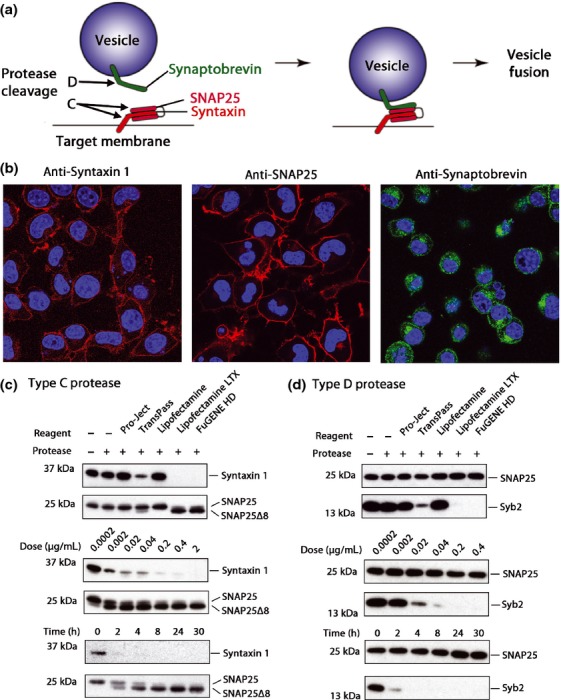
Efficient transduction of botulinum type C and D proteases into neuroblastoma cells. (a) Schematic of the complex formation by synaptobrevin, v-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) (green) and syntaxin/SNAP25, t-SNAREs (red and fuchsia) necessary for vesicle fusion with the cell membrane. The substrates of the botulinum protease type C are syntaxin and SNAP25, whereas the type D protease cleaves only synaptobrevin. (b) Confocal image showing syntaxin 1 (red; left), SNAP25 (red; middle), and synaptobrevins 1-3 (green; right) in Neuro2A cells as revealed by immunocytochemistry. (c) Comparison of the efficiency of different reagents used to deliver botulinum protease type C (top panel). Cleavage of syntaxins evidenced by the disappearance of the immunoreactive band, whereas cleavage of SNAP25 is evidenced by the appearance of a proteolytic product SNAP25Δ8. SNAP25 immunoreactivity served as a loading control. Middle panel shows the dose dependence of SNARE cleavage following 20 h treatment. Bottom panel shows a time course using 0.2 μg/mL of the type C protease. (d) Comparison of different reagents used to deliver botulinum protease type D (top panel) and efficacy (lower panels) of synaptobrevin 2 (Syb2) cleavage evidenced by the disappearance of the immunoreactive band. SNAP25 was used as a loading control.
