Fig. 5.
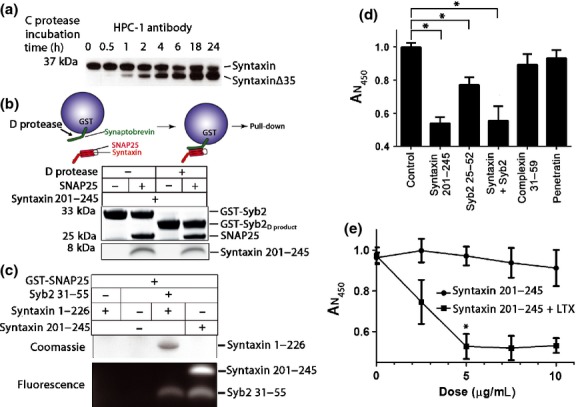
Shorted soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) fragments can interact and cause cytotoxicity upon transduction. (a) Western immunoblotting of N2A cells, treated with 0.002 μg of type C protease for up to 24 h, reveals cleaved syntaxin fragment 1-253 (syntaxinΔ35). In this instance immunoblotting was performed using the HPC-1 anti-syntaxin antibody. (b) Schematic of a SNARE pull-down reaction (upper panel). The lower panel showing cleavage of Glutathione S-transferase (GST)-synaptobrevin 2 (GST-Syb2) by type D protease and the ability of this product to bind syntaxin fragment (201-245) and SNAP25 as seen in the Coomassie-stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) gel. Note SNAP25 is required for the syntaxin pull-down by GST-Syb2 and GST-Syb2D product highlighting the ternary nature of the SNARE interaction. (c) GST-SNAP25 pull-down experiment reveals interaction of the indicated shortened synaptobrevin and syntaxin fragments, strictly in a ternary manner. Syntaxin fragment 1-226 incorporating the regulatory syntaxin head with a truncated SNARE motif was revealed by Coomassie staining, whereas short SNARE peptides, carrying FITC, were imaged in the Bio-Rad XRS imaging station. (d) Bar chart showing cell survival measured using CCK-8 assay. Intracellular delivery of syntaxin fragment (201-245) and synaptobrevin (25-52) peptide using Lipofectamine LTX causes a significant reduction in cell viability, whereas transduction of control peptides, complexin (31-59) and penetratin, at the same concentration does not lead to cell death (*p < 0.05). Results are normalized to control and presented ± SD. (e) The short syntaxin fragment (201-245) applied to N2A cells causes cell death in a dose-dependent manner. Changes in cell viability are shown with and without Lipofectamine LTX. A significant loss of viability can be observed at doses as low as 5 μg/mL (*p < 0.01, ± SD).
