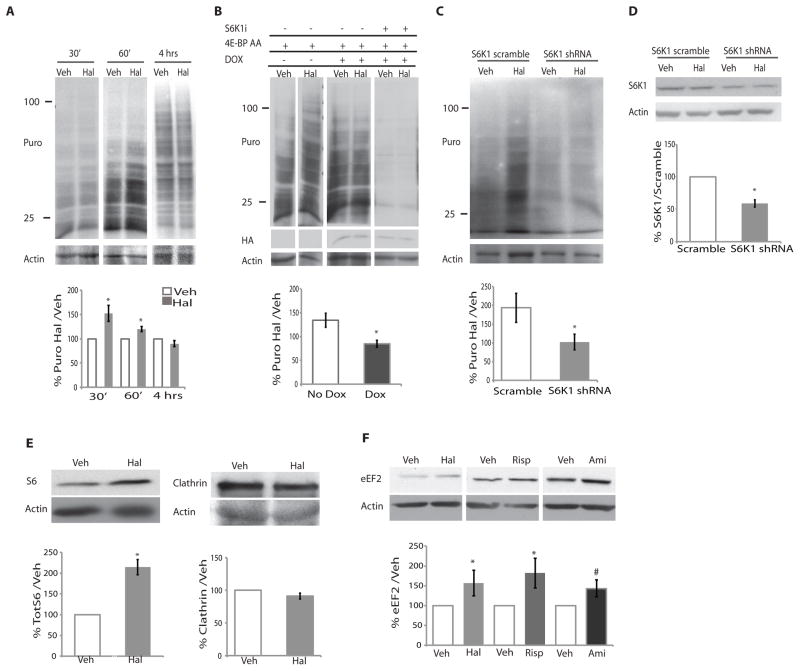
Figure 2. Acute haloperidol treatment leads to transient increases in protein synthesis that are mTORC1 dependent.
(A) Primary DIV7 striatal neurons were treated with haloperidol (Hal) or DMSO (Veh) for 30, 60 minutes, or 4 hours and 1 μg/ml puromycin. Puromycin was detected by Western blotting. A representative blot is shown. The 4-hr blot is a lighter exposure due to the intensity of the signal. Data were quantified as the percent of puromycin signal in the haloperidol-treated samples / puromycin signal in vehicle–treated samples normalized to actin and shown as the average (30 min; n=8, p =0.007; 60 min, n=7, p=0.005; 4 hours, n=4, not significant). Actin is a loading control. (B) The effect of S6K1 shRNA or the doxycycline-inducible (DOX) dominant-negative 4E-BP AA on haloperidol-mediated stimulation of translation. Data were quantified as in panel A for the 4E-BP AA “on” (Dox; n=4) and “off” (No Dox; n=3) conditions. * p=0.02). Doxycycline with and without S6K1 inhibitor (S6K1i), n = 3, not quantified due to comparatively light signal. HA indicates the presence of 4E-BP AA. Actin is a loading control. (C) The effect of S6K1 knockdown (S6K1 shRNA) or control (S6K1 scramble) on the haloperidol-induced increase in puromocyin incorporation. Data are quantified as in panel A. (n=4, p=0.03,). (D) Effectiveness of S6K1 knockdown was quantified for each experiment, knockdown average was 41% (n= 4 p < 0.0003). (E) Western blot analysis of S6 and clathrin abundance in striatal neurons exposed to vehicle or haloperidol under the same conditions used for the puromycin analysis (% Haloperidol/Vehicle normalized to actin loading control). (S6, n=3, p=0.003) (clathrin, n=4, p=not signficant). (F) Western blot analysis of eEF2 abundance in striatal neurons exposed to vehicle or haloperidol (20 μM, n=7, p=0.027), risperidone (Risperidone 100 nM, n=,5, p < 0.06), or amisulpride (Ami, 1 μM, n = 5, p = 0.08) for 4 hours. All graphs shown are average ± SEM and analyzed by Student’s t test. P < 0.05 was considered significant and is marked with asterisks; P< 0.1 is marked with #.