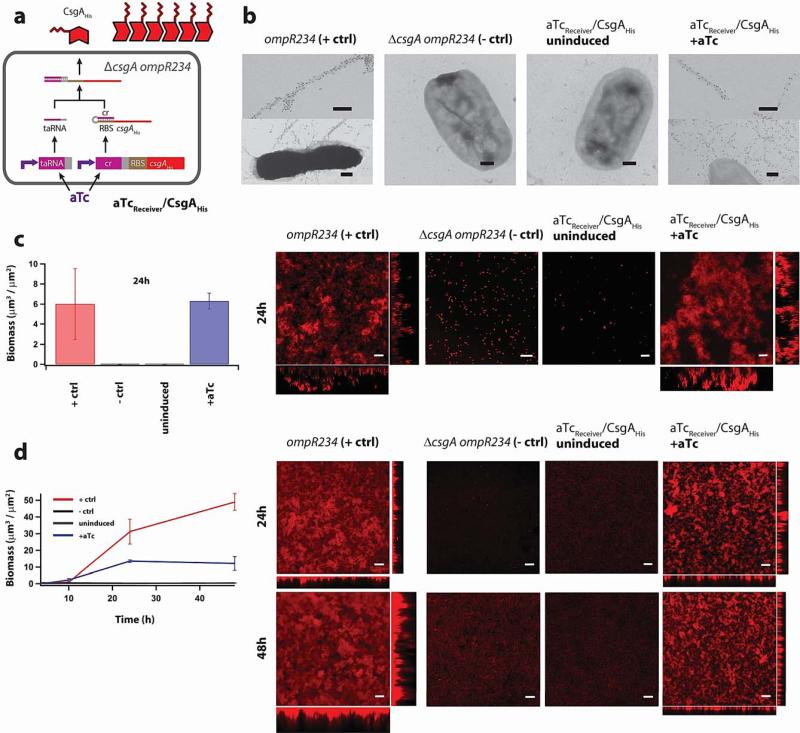
Figure 1. Inducible production of engineered curli fibrils and biofilms.
a, Riboregulator circuits tightly regulate expression of curli subunits, such as CsgAHis. Production of CsgAHis requires the expression of trans-activating RNA (taRNA). The taRNA prevents the cis-repressive (cr) sequence from blocking the ribosome-binding sequence (RBS) controlling translation of the mRNA transcript. In the absence of inducer, mRNA and taRNA levels are low, thus leading to significant repression of gene expression. The addition of aTc induces transcription of both csgAHis mRNA and taRNA, thus enabling CsgAHis production. Tight regulation of curli expression is useful for controlling patterning (Supplementary Fig. 19). b, Immuno-labelling of curli fibrils with rabbit anti-CsgA antibodies and gold-conjugated goat anti-rabbit antibodies. Positive-control (“+ ctrl”) MG1655 ompR234 cells (“ompR234”, see Supplementary Table 3), which have an intact endogenous csgA gene, produce curli fibrils that were labelled by anti-CsgA antibodies and are attached to cells. However, negative-control (“− ctrl”) cells with the csgA gene knocked out and no csgA-expressing circuits (“ΔcsgA ompR234”, see Supplementary Table 3), as well as aTcReceiver/CsgAHis cells in the absence of aTc, did not produce curli fibrils. Inducing aTcReceiver/CsgAHis cells with aTc enabled the synthesis of curli fibrils that were labelled by anti-CsgA antibodies and attached to cells. Scale bars are 200nm. c, Confocal microscopy and biomass quantification revealed that under static culture conditions, E. coli ompR234 cells formed thick adherent biofilms. However, E. coli ΔcsgA ompR234 cells, as well as aTcReceiver/CsgAHis cells in the absence of aTc, did not form biofilms. Inducing aTcReceiver/CsgAHis cells with aTc led to the formation of thick adherent biofilms. d, Confocal microscopy and biomass quantification revealed similar biofilm-forming capabilities by E. coli ompR234 and induced aTcReceiver/CsgAHis cells when grown in flow cells. To enable visualization, we transformed a constitutive mCherry-expressing plasmid into all strains (see Supplementary Methods). Cells were grown in liquid M63 media with glucose; the corresponding experiments for other media conditions are shown in Supplementary Figure 1 and 2. Scale bars in c) and d) are 50μm, and orthogonal XZ and YZ views are maximum-intensity projections.