Abstract
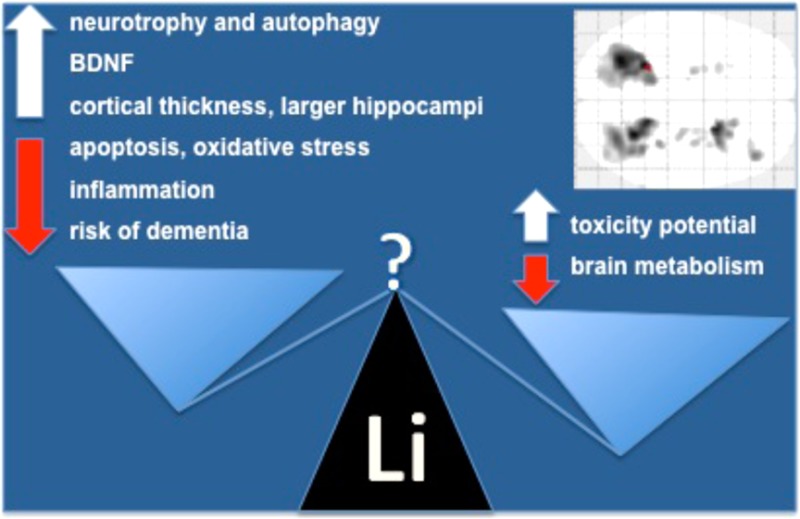
Lithium modulates several intracellular pathways related to neuroplasticity and resilience against neuronal injury. These properties have been consistently reported in experimental models, and involve the up-regulation of neurotrophic response and autophagy, and down-regulation of apoptosis, oxidative stress, and inflammation. Clinical and epidemiological studies in bipolar disorder show that acute treatment with lithium increases plasma concentrations of brain-derived neurotrophic factor, and long-term treatment lowers the risk of dementia. Neuroimaging studies indicate that lithium use is further associated with increased cortical thickness and larger hippocampal volumes. The objective of the present study was to evaluate whether these neurobiological properties of lithium reflect in increased regional brain glucose metabolism, as shown by [18F]FDG-PET. Participants (n = 19) were nondemented older adults recruited at the end point of a controlled trial addressing clinical and biological effects of lithium in a sample of patients with amnestic mild cognitive impairment. Twelve patients who had received low-dose lithium carbonate for 4 years were compared to seven matched controls. Chronic lithium treatment was not associated with any significant increase in brain glucose metabolism in the studied areas. Conversely, we found a significant reduction in glucose uptake in several clusters of the cerebellum and in both hippocampi. These findings were not associated with any clinical evidence of toxicity. The clinical implications of the present findings need to be clarified by future controlled studies, particularly in the light of the potential use of lithium as a disease-modifying treatment approach for certain neurodegenerative disorders, namely, Alzheimer’s disease and amyotrophic lateral sclerosis.
Keywords: Lithium, brain metabolism, [18F]FDG-PET, mild cognitive impairment
Lithium salts remain an important therapeutic option in clinical psychiatry, particularly for the treatment of bipolar disorder and as adjunctive therapy for major depression. The clinical benefits of lithium in mood disorders are unequivocal, acting as a mood stabilizer and reducing both suicide and mortality in the long term.1−3 More recently, translational research provided evidence of the neuroprotective properties of lithium (for a review, see Forlenza et al.),4 yielding the rationale for its clinical testing as a disease-modifying therapy for certain neurodegenerative disorders, namely, amyotrophic lateral sclerosis (ALS),5 Alzheimer’s disease (AD),6,7 and its prodromal stage clinically defined as amnestic mild cognitive impairment (aMCI).8 In clinical grounds, chronic lithium use has been associated with a lower prevalence of dementia,9 particularly among patients with bipolar disorder.10,11 This potential neuroprotective effect was also associated with the up-regulation of neurotrophic cascades12−14 and reduction of inflammatory response and oxidative stress.15−18 Therefore, the putative neuroprotective effects of lithium in the MCI-AD continuum may result both from unspecific neurobiological mechanisms (aforementioned) and from specific effects on biochemical pathways that are central to the pathogenesis of AD, such as the inhibition of glycogen synthase-kinase 3 beta (GSK-3B), up-regulation of autophagy, and down-regulation of apoptosis.4
Structural neuroimaging studies showed that both short and long-term lithium treatment were associated with increased cortical thickness and volumetric increase in the hippocampus and amygdala of bipolar patients.19−23 In addition, studies with magnetic resonance spectroscopy showed increased N-acetyl aspartate (NAA) and myo-inositol levels after lithium treatment in subjects with bipolar disorder.24,25 Very few studies have addressed the effects of lithium on brain glucose metabolism with PET scans. Tonn et al. reported a case of lithium intoxication associated with AD-like changes in temporoparietal areas, suggesting an overlap of clinical and biological manifestations in these two conditions.26 Hollander et al. conducted an [18F]FDG-PET study in pathological gamblers, showing that lithium increases orbitofrontal, dorsolateral, and cingulate metabolism.27 The objective of the present study is to evaluate the differences in regional brain glucose metabolism with [18F]FDG-PET in elderly adults with aMCI patients after a 4 year treatment with lithium chloride versus placebo.
Results and Discussion
Table 1 displays clinical and demographic data of participants. There were no statistically significant differences in age (p = .398), education (p = .251) or gender distribution (p = .211) between the two groups. Also, no significant differences in global cognitive performance were found (p = .376). All patients presented a normal score (nondementia range) in the Mini-Mental State Examination (MMSE). In a first exploratory analysis, no significant increases in regional blood glucose metabolism (rBGM) were found in the lithium group in relation to the placebo group. When looking for rBGM reductions among lithium users, a reduction in several clusters in the cerebellum was found and corrected for multiple comparisons. rBGM reductions in several peak voxels were also observed, but only in uncorrected analyses. No peak voxel remained significant after multiple comparisons (Table 2).
Table 1. Clinical and Demographic Characteristics of the Participants.
| lithium (n = 12) | placebo (n = 7) | p | |
|---|---|---|---|
| age, yearsa | 75.2 (6.13) | 77.8 (4.07) | 0.398b |
| education, yearsa | 8.5 (5.82) | 11.14 (3.84) | 0.251b |
| female sex (n) | 10 | 4 | 0.211c |
| MMSE | 26.8 (2.62) | 27.7 (1.60) | 0.376b |
Mean (standard deviation); MMSE = Mini-Mental State Examination.
t test.
χ2 test.
Table 2. Regional Brain Glucose Metabolism (rBGM) Comparison between Lithium and Placebo Groups Using SPM8a.
| pFWE (cluster level) | pFDR (cluster level) | Z score (peak level) | punc (peak level) | peak voxel (Talairach) | |
|---|---|---|---|---|---|
| lithium group × control group (reduction) | |||||
| cluster 1 (1713 voxels) | 0.000 | 0.000 | |||
| left cerebellum, anterior lobe | 4.60 | 0.000 | –26, −46, −34 | ||
| left cerebellum, posterior lobe, inferior semi-lunar lobule | 4.51 | 0.000 | –30, −64, −37 | ||
| left cerebellum, posterior lobe, cerebellar tonsil | 4.12 | 0.000 | –32, −50, −44 | ||
| cluster 2 (1395 voxels) | 0.000 | 0.000 | |||
| right cerebellum, posterior lobe, cerebellar tonsil | 4.54 | 0.000 | 26, −54, −38 | ||
| right cerebellum, posterior lobe, cerebellar tonsil | 4.33 | 0.000 | 18, −46, −35 | ||
| right cerebellum, posterior lobe, cerebellar tonsil | 0.000 | 28, −68, −40 | |||
| cluster 3 (197 voxels) | 0.051 | 0.025 | |||
| right cerebrum, temporal lobe, superior temporal gyrus | 4.40 | 0.000 | 22, 8, −41 | ||
| right cerebrum, temporal lobe, superior temporal gyrus | 3.95 | 0.000 | |||
| right cerebrum, frontal lobe, inferior frontal gyrus | 3.80 | 0.000 | |||
| control group × lithium group (reduction) | |||||
|---|---|---|---|---|---|
| no significative changes in rBGM corrected for multiple comparisons |
Results shown at the cluster and voxel levels (global analysis, maps vizualized with punc < 0.001). Nonpaired t test, p values corrected for multiple comparisons (FWE, familywise error method; FDR, false discovery rate method) and uncorrected at the peak voxel level (punc).
Small volume correction (SVC) analyses of the cerebellum and areas related to cognition indicated significant reductions in brain metabolism in several voxels in the cerebellum and both hippocampi among lithium treated patients; these differences remained significant after correction for multiple comparisons (Tables 3 and 4).
Table 3. rBGM Reductions at the Peak Level in the Lithium Group in Comparison to the Placebo Groupa.
| pFWEb | pFDRb | Z score | peak voxel (Talairach) | |
|---|---|---|---|---|
| left cerebellum, posterior lobe, inferior semi-lunar lobule | 0.007 | 0.055 | 4.48 | –30, −64, −39 |
| left cerebellum, posterior lobe, cerebellar tonsil | 0.013 | 0.055 | 4.31 | –30, −52, −33 |
| left cerebellum, anterior lobe, culmen | 0.013 | 0.055 | 4.30 | –28, −48, −26 |
| left cerebellum, anterior lobe, culmen | 0.016 | 0.061 | 4.24 | –22, −60, −26 |
| left cerebellum, posterior lobe, cerebellar tonsil | 0.031 | 0.088 | 4.04 | –18, −44, −33 |
| right cerebellum, posterior lobe, cerebellar tonsil | 0.009 | 0.122 | 4.42 | 18, −46, −35 |
| right cerebellum, posterior lobe, cerebellar tonsil | 0.013 | 0.122 | 4.31 | 25, −54, −39 |
| right cerebellum, posterior lobe, pyramis | 0.033 | 0.152 | 4.04 | 28, −68, −30 |
| right cerebellum, anterior lobe, culmen | 0.043 | 0.163 | 3.95 | 38, −48, −30 |
Small volume correction analysis directed to cerebellum as area of interest (maps generated for analysis with a minimum threshold of p < 0.001, uncorrected for multiple comparisons).
Nonpaired t test; differences in rBGM (p values) were considered as significant only after correction for multiple comparisons (FWE, familywise error; FDR, false discovery rate).
Table 4. rBGM Reductions at the Peak Level in the Lithium Group in Comparison to the Placebo Groupa.
| pFWE | Z score | peak voxel (Talairach) | |
|---|---|---|---|
| right cerebrum, temporal lobe, sub-gyral, gray matter, hippocampus | 0.018 | 3.73 | 32, −33, −5 |
| right cerebrum, limbic lobe, parahippocampal gyrus, gray matter, hippocampus | 0.041 | 3.46 | 26, −18, −13 |
| left cerebrum, limbic lobe, parahippocampal gyrus, gray matter, amygdala | 0.039 | 3.47 | –22, −8, −18 |
Small volume correction analysis directed to areas previously related to neurodegeneration in aMCI. Nonpaired t test; p values corrected for multiple comparisons (FWE- familywise error).
To the best of our knowledge, this is the first controlled study to address the effects of chronic lithium treatment on brain glucose metabolism with the aid of [18F]FDG imaging. We found that long-term exposure to lithium at subtherapeutic levels (0.25–0.5 mEq/L) was associated with a relative decrease in brain glucose metabolism affecting numerous areas of the cerebellum. This imaging finding was not associated with any clinically relevant signs of toxicity, or with complaints suggestive of side effects. Anyhow, these findings could be indicative of subclinical toxicity related to the cerebral effect of the drug, particularly taking into account the older age of participants.
Lithium toxicity has been previously reported in the literature, mostly in individuals using therapeutic doses at the upper recommended limit (0.8–1.1 mEq/L), or as a consequence of acute intoxication. Although relatively uncommon, cerebellar toxicity is a potentially severe and sometimes irreversible complication of lithium use, particularly in the elderly. It usually presents with dysarthria and speech impairment, intention tremor, ataxia, and may have an acute, subacute, or chronic onset.28 The Syndrome of Irreversible Lithium-Effectuated Neurotoxicity (known as SILENT syndrome) may also be associated with encephalopathy and cognitive abnormalities.29,30 Some of the patients may further develop cerebellar atrophy, which can be depicted by structural neuroimaging (MRI/CT),31 and reduction of cerebellar glucose metabolism as shown by [18F]FDG-PET scans.30 In addition, neuropathological studies of the human brain and experimental models indicated that lithium-induced neurotoxicity is associated with a selective loss of Purkinje cells, white and gray matter vacuolization, and astrocitosis.32−34 Therefore, the present findings raise an important safety issue regarding a potential adverse event associated with the chronic administration of lithium to older patients. Even though (as previously said) none of the patients in the present sample had any clinical signs of lithium toxicity, the reduction in cerebellar glucose metabolism may be an early event leading to dysfunction, which may require a more detailed and active investigation of subtle clinical signs. Future studies with appropriate methodology are definitely needed to address this possibility.
Another intriguing result of the present analysis (less prominent, but nonetheless significant) was the bilateral reduction of [18F]FDG uptake in the hippocampus of lithium-treated patients. This finding was, in fact, quite opposite to our a priori hypothesis, that is, that lithium might increase hippocampal metabolism, as a consequence of the up-regulation of biological responses associated with mitochondrial function,35 neurotrophic response,12,14 and neurogenesis.36,37 Not only do our data not support this effect, but rather there seems to be a bilateral reduction in glucose consumption in this area, suggestive of hippocampal hypometabolism. In a case report presented by Tonn et al., a 75 year old senior who had been treated with lithium for decades and developed acute (AD-like) cognitive changes during an episode of lithium intoxication was scanned with [18F]FDG-PET.26 A marked bilateral reduction in glucose metabolism was observed in temporoparietal areas, in a similar pattern to that typically observed in AD. Interestingly, both cognitive symptoms and rBGM abnormalities reversed to normal after withdrawal of the drug and clearance of the intoxication. The authors concluded that lithium toxicity might disturb brain glucose metabolism in specific areas that are also affected in AD, inducing an AD-like presentation reinforced both by functional neuroimaging and neuropsychological findings. Therefore, we understand that this report is in line with our perception that lithium may actually induce some form of hippocampal toxicity.
Although most practice guidelines recommend lithium as a first-line drug for the long-term treatment of bipolar disorder, its use is not devoid of safety concerns.38 In fact, there are many potential adverse events related to the prescription of the drug, ranging from a relatively high incidence of unpleasant but not life threatening side effects (e.g., tremor, polyuria, weight gain) to the risk of irreversible organ damage such as renal failure and hypothyroidism, and neurological syndromes secondary to lithium intoxication.39 Therefore, there is need for controlled observations of the potential lithium toxicity upon long-term use, particularly in the elderly.40
Several methodological aspects must be taken into account in the interpretation of the findings, namely: (i) the small number of cases (lithium-treated) and controls (placebo group); (ii) the older age of participants; and (iii) the fact that cases and controls, although clinically healthy and nondemented, had memory symptoms compatible with the diagnosis of aMCI, which is a well-established risk factor for AD. Therefore, abnormalities affecting medial temporal regions may have been present since the beginning of the trial in a proportion of cases and controls, but distributed heterogeneously in both groups. In this case, the progression of brain changes (including rBGM) in the subsample of aMCI patients with prodromal AD might be unevenly distributed, introducing a confounding factor to our case control data that could not be ruled out with the present methodology. Ideally, a baseline assessment with [18F]FDG-PET should be used to test for group-specific modifications in brain glucose metabolism at end point. This exploration was beyond the original objectives of the clinical trial and, therefore, a baseline PET assessment was not included in the protocol. Therefore, we consider our results preliminary and reinforce the need for adequately powered and controlled studies to address this possibility in further depth. Nonetheless, the clinical and biological implications of this effect, if confirmed, will be undoubtedly relevant to the long-term management of bipolar disorder and will raise concerns about the safety of lithium use as a neuroprotective agent.
Conclusion
We investigated the modifications of regional blood glucose metabolism induced by long-term lithium treatment in a sample of older adults with aMCI. We did not find any evidence of increase in the uptake of [18F]FDG-PET; rather, lithium treated patients (as compared to those in the placebo group) had significantly lower glucose uptake in several areas of the cerebellum and, less prominently, both hippocampi. Our data do not support the notion that the neurotrophic response associated with the effect of lithium in experimental models and in humans are associated with an increase in regional brain metabolism. Conversely, chronic lithium use may be associated with an opposite effect, particularly in the cerebellum and medial temporal lobe structures. The clinical implications of this effect still need to be elucidated.
Methods
Participants and Study Design
The present study is part of a single-center, randomized, placebo-controlled trial to assess the potential neuroprotective effects of chronic, low-dose, lithium treatment in nondemented older adults with aMCI conducted at the Institute of Psychiatry, Faculty of Medicine, University of São Paulo.8 Participants were community-dwelling outpatients recruited from the hospital catchment area.41 At the screening phase, global cognitive performance was ascertained with the aid of the Mini-Mental State Examination (MMSE), and cases of dementia were ruled out according to education-adjusted cutoff scores (≥26 for subjects with at least 8 years of formal education and ≥24 for those with lower education levels but not illiterate). A total of 76 subjects meeting inclusion criteria were recruited to the present study after signing informed consent. Almost all patients were Caucasian (95%) with normal body mass index. Mean serum concentrations of lithium were 0.38 mEq/L with a standard deviation of 0.19 mEq/L. Inclusion criteria were as follows: age 60 years or older; diagnosis of aMCI according to Mayo Clinic criteria42 and no evidence of relevant clinical and psychiatric disorders. All patients were submitted to a broad neuropsychological evaluation and to the ADAS-Cog battery (for details, please see Diniz et al.41 and Forlenza et al.8). After initial assessment, 15 patients declined participation and the remaining 61 were randomized to receive lithium or placebo and were longitudinally assessed for clinical, cognitive and biological outcomes at 3 month intervals. The first 2 years of treatment was conducted in a double-blind model, and the extension phase (years 3 and 4) was single-blinded. This intervention study and its substudies were approved by the local Ethics Committee and conducted in adherence with the Helsinki Declaration and Good Clinical Practice recommendations. The intervention trial was registered in www.clinicaltrials.gov (NCT01055392).
We present here the results of a case-control analysis of a cross section of completers of the aforementioned intervention trial, addressing the long-term modifications to regional brain glucose metabolism (rBGM) after of 4 years of continuous lithium use. Nineteen participants (12 subjects from the lithium group and 7 from the placebo group) were referred to the Nuclear Medicine Center (at the same institution) to perform [18F]FDG-PET imaging of the brain.
Lithium Dose Titration
Identical tablets containing 150, 300, 450, or 600 mg of lithium carbonate or placebo stored in packages with identical coded blisters were produced at the hospital pharmacy. Throughout treatment and after each weekly visit, participants received from the pharmacist two batches of cases containing either lithium or placebo tablets. Blisters were identified for use in the morning and/or at bedtime, and patients were instructed to take one tablet q.d. or b.i.d., preferably with meals, following the prescription from the main physician investigator (O.V.F.). Therefore, it was possible to titrate the daily doses of lithium or to maintain the placebo regimen by regulating the combinations of tablets.
After randomization, patients in the lithium group were started on daily doses of 150 mg of lithium, which was titrated weekly to target serum levels. In case of relevant side-effects during the titration phase, the lithium dose was adjusted back to the highest tolerable dose within the treatment range. Serum lithium levels were determined weekly in the titration phase, 12 h after the last dose. The target lithium range defined was 0.25–0.5 mmol/L to minimize the risk of adverse events, and to reduce the rate of discontinuation due to side-effects (for more information, please see previous study information; Forlenza et al.8).
Once stable lithium levels were achieved, the prescription was maintained until the next visit, which was scheduled at 3 month intervals. Patients were instructed to report any adverse events or modifications made to other ongoing prescriptions; if necessary, the maintenance of lithium treatment was to be re-evaluated.
[18F]FDG-PET Imaging
All subjects gave their written informed consent prior to imaging examination. To ensure blood glucose levels lower than 180 mg/mL, subjects fasted for at least 4 h prior to an intravenous injection of 185 MBq of [18F]FDG in a peripheral vein. After the tracer injection, patients rested with eyes open and ears unplugged for 60 min in a calm, silent, and slightly darkened room. Acquisition was performed for 15 min (matrix = 256 × 256, zoom = 2.5, pixel size = 1.04), using a Siemens Biograph PET-CT scanner (CTI/Siemens, Knoxville, TN). Images were reconstructed with the iterative OSEM method (6 interactions, 16 subsets) and then smoothened with a 5 mm Gaussian filter. Data were also corrected for scattering, attenuation and decay. No partial volume correction was applied. Attenuation correction was performed using a single helical computed tomography.
Statistical Analysis
A voxel-based analysis of the PET data was performed using the SPM8 software (Wellcome Department of Cognitive Neurology, Functional Imaging Laboratory, London, U.K.). A nonpaired t test was used to examine differences between groups. Details of the processing are shown below. Anatomic normalization and statistical processing for [18F]FDG-PET was performed using SPM8 in conjunction with the MATLAB R2009a (The Mathworks Inc.). All PET images were spatially normalized in SPM8 to a standard stereotactic space based on the Talairach and Tournoux atlas. Each of the scans was also individually smoothed with a Gaussian kernel to reduce the impact of misregistration into template space and to improve the signal-to-noise ratio. Images were then smoothed with a 4 mm full-width at half-maximum Gaussian filter. To ensure that the analysis only included voxels mapping cerebral tissue, a default threshold of 80% of the mean uptake inside the brain was selected. Global uptake differences between brain scans were adjusted using the “proportional scaling” SPM option. As previously described, a nonpaired t test analysis was used to examine metabolic differences across Lithium and Placebo groups (two-sample paired t test converted to z scores). Each pairwise analysis was performed in both positive and negative directions (searching for metabolic increases or decreases in the lithium group), to observe changes in rBGM. SPM8 maps were generated with uncorrected p values at the peak voxel level (punc) < 0.01. The threshold for significance both at the cluster and voxel cluster levels was set at p = 0.05, corrected for multiple comparisons using both the false discovery rate (FDR) and the family wise error (FWE) statistic methods, and a minimum cluster size of 10 voxels was selected. The relevant peak voxels were identified in terms of coordinates according to Talairach and Tournoux with the help of the Talairach Client software, after conversion from the SPM8 space.43,44
Taking into account the previously related cerebellar toxicity of lithium in higher doses, a directed analysis addressing the cerebellum was also performed with the SVC method. SVC analyses were also performed in several areas related to cognition and neurodegeneration in aMCI: posterior cingulate temporoparietal areas, hippocampi and parahippocampal regions.
Author Contributions
O.V. Forlenza designed and wrote the paper; A.M.N. Coutinho evaluated PET scans and wrote the paper; I. Aprahamian evaluated the sample and wrote the paper; S. Prando evaluated PET scans; L.L. Mendes evaluated PET scans; B.S. Diniz evaluated the sample; W.F. Gattaz revised the paper and project design; C.A. Buchpiguel discussed the PET scans and revised the paper.
FAPESP (2009/52825-8), Alzheimer’s Association (NIRG-08-90688), Associação Beneficente Alzira Denise Hertzog da Silva (ABADHS).
The authors declare no competing financial interest.
References
- Geddes J. R.; Burgess S.; Hawton K.; Jamison K.; Goodwin G. M. (2004) Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am. J. Psychiatry 161, 217–222. [DOI] [PubMed] [Google Scholar]
- Cipriani A.; Pretty H.; Hawton K.; Geddes J. R. (2005) Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am. J. Psychiatry 162, 1805–1819. [DOI] [PubMed] [Google Scholar]
- Ohgami H.; Terao T.; Shiotsuki I.; Ishii N.; Iwata N. (2009) Lithium levels in drinking water and risk of suicide. Br J. Psychiatry 194, 464–465. [DOI] [PubMed] [Google Scholar]
- Forlenza O. V.; de Paula V. J.; Machado-Vieira R.; Diniz B. S.; Gattaz W. F. (2012) Does lithium prevent Alzheimer’s disease?. Drugs Aging 29, 335–342. [DOI] [PubMed] [Google Scholar]
- Chiò A.; Mora G. (2012) The final chapter of the ALS lithium saga. Lancet Neurol. 12, 324–325. [DOI] [PubMed] [Google Scholar]
- Macdonald A.; Briggs K.; Poppe M.; Higgins A.; Velayudhan L.; Lovestone S. (2008) A feasibility and tolerability study of lithium in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 23(7), 704–711. [DOI] [PubMed] [Google Scholar]
- Hampel H.; Ewers M.; Bürger K.; Annas P.; Mörtberg A.; Bogstedt A.; Frölich L.; Schröder J.; Schönknecht P.; Riepe M. W.; Kraft I.; Gasser T.; Leyhe T.; Möller H. J.; Kurz A.; Basun H. (2009) Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J. Clin. Psychiatry 70(6), 922–931. [PubMed] [Google Scholar]
- Forlenza O. V.; Diniz B. S.; Radanovic M.; Santos F. S.; Talib L. L.; Gattaz W. F. (2011) Disease-modifying properties of long-term lithium treatment for amnestic mild cognitive impairment: randomised controlled trial. Br J. Psychiatry 198, 351–356. [DOI] [PubMed] [Google Scholar]
- Terao T.; Nakano H.; Inoue Y.; Okamoto T.; Nakamura J.; Iwata N. (2006) Lithium and dementia: a preliminary study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 30, 1125–1128. [DOI] [PubMed] [Google Scholar]
- Nunes P. V.; Forlenza O. V.; Gattaz W. F. (2007) Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br J. Psychiatry 190, 359–360. [DOI] [PubMed] [Google Scholar]
- Kessing L. V.; Forman J. L.; Andersen P. K. (2010) Does lithium protect against dementia?. Bipolar Disord. 12, 87–94. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R.; Manji H. K.; Zarate C. A. Jr (2009) The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 11(Suppl 2), 92–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwalska A.; Sobieska M.; Rybakowski J. K. (2010) Serum brain-derived neurotrophic factor in euthymic bipolar patients on prophylactic lithium therapy. Neuropsychobiology 62, 229–234. [DOI] [PubMed] [Google Scholar]
- Rybakowski J. K.; Suwalska A. (2010) Excellent lithium responders have normal cognitive functions and plasma BDNF levels. Int. J. Neuropsychopharmacol. 13, 617–622. [DOI] [PubMed] [Google Scholar]
- Knijff E. M.; Breunis M. N.; Kupka R. W.; de Wit H. J.; Ruwhof C.; Akkerhuis G. W.; Nolen W. A.; Drexhage H. A. (2007) An imbalance in the production of IL-1beta and IL-6 by monocytes of bipolar patients: restoration by lithium treatment. Bipolar Disord. 9, 743–753. [DOI] [PubMed] [Google Scholar]
- Guloksuz S.; Altinbas K.; Aktas Cetin E.; Kenis G.; Bilgic Gazioglu S.; Deniz G.; Oral E. T.; van Os J. (2012) Evidence for an association between tumor necrosis factor-alpha levels and lithium response. J. Affective Disord. 143, 148–152. [DOI] [PubMed] [Google Scholar]
- Khairova R.; Pawar R.; Salvadore G.; Juruena M. F.; de Sousa R. T.; Soeiro-de-Souza M. G.; Salvador M.; Zarate C. A.; Gattaz W. F.; Machado-Vieira R. (2012) Effects of lithium on oxidative stress parameters in healthy subjects. Mol. Med. Rep. 5, 680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa R. T.; Zarate C. A. Jr; Zanetti M. V.; Costa A. C.; Talib L. L.; Gattaz W. F.; Machado-Vieira R. (2014) Oxidative stress in early stage Bipolar Disorder and the association with response to lithium. J. Psychiatr. Res. 50, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden C. E.; Thompson P. M.; Dalwani M.; Hayashi K. M.; Lee A. D.; Nicoletti M.; Trakhtenbroit M.; Glahn D. C.; Brambilla P.; Sassi R. B.; Mallinger A. G.; Frank E.; Kupfer D. J.; Soares J. C. (2007) Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol. Psychiatry 62, 7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. J.; Cortese B. M.; Glitz D. A.; Zajac-Benitez C.; Quiroz J. A.; Uhde T. W.; Drevets W. C.; Manji H. K. (2009) A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J. Clin. Psychiatry 70, 699–705. [DOI] [PubMed] [Google Scholar]
- Lyoo I. K.; Dager S. R.; Kim J. E.; Yoon S. J.; Friedman S. D.; Dunner D. L.; Renshaw P. F. (2010) Lithium-induced gray matter volume increase as a neural correlate of treatment response in bipolar disorder: a longitudinal brain imaging study. Neuropsychopharmacology 35, 1743–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Erp T. G.; Thompson P. M.; Kieseppä T.; Bearden C. E.; Marino A. C.; Hoftman G. D.; Haukka J.; Partonen T.; Huttunen M.; Kaprio J.; Lönnqvist J.; Poutanen V. P.; Toga A. W.; Cannon T. D. (2012) Hippocampal morphology in lithium and non-lithium-treated bipolar I disorder patients, non-bipolar co-twins, and control twins. Hum. Brain Mapp. 33, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T.; Calkin C.; Blagdon R.; Slaney C.; Alda M. (2013) Type 2 Diabetes Mellitus: A Potentially Modifiable Risk Factor for Neurochemical Brain Changes in Bipolar Disorders. Biol. Psychiatry 10.1016/j.biopsych.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Silverstone P. H.; Wu R. H.; O’Donnell T.; Ulrich M.; Asghar S. J.; Hanstock C. C. (2003) Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetyl-aspartate concentrations in euthymic bipolar patients. Int. Clin Psychopharmacol 18, 73–79. [DOI] [PubMed] [Google Scholar]
- Forester B. P.; Finn C. T.; Berlow Y. A.; Wardrop M.; Renshaw P. F.; Moore C. M. (2008) Brain lithium, N-acetyl aspartate and myo-inositol levels in older adults with bipolar disorder treated with lithium: a lithium-7 and proton magnetic resonance spectroscopy study. Bipolar Disord. 10, 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn P.; Bartensten P.; Dahmen N. (2005) Lithiumintoxikation imitiert Alzheimer-Demenz in PET und klinischen Befund. Nervenarzt 76, 613–616. [DOI] [PubMed] [Google Scholar]
- Hollander E.; Buchsbaum M. S.; Haznedar M. M.; Berenguer J.; Berlin H. A.; Chaplin W.; Goodman C. R.; LiCalzi E. M.; Newmark R.; Pallanti S. (2008) FDG-PET study in pathological gamblers: lithium increases orbitofrontal, dorsolateral and cingulate metabolism. Neuropsychobiology 58, 37–47. [DOI] [PubMed] [Google Scholar]
- Von Hartitzsch B.; Hoenisch N. A.; Leigh R. J.; Wilkinson R.; Frost T. H.; Weddel A.; Posen G. A. (1972) Permanent neurological sequelae despite haemodialysis for lithium intoxication. BMJ [Br. Med. J.] 4, 757–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aditianjee; Munshi K. R.; Thampy A. (2005) The syndrome of irreversible lithium-effectuated neurotoxicity. Clin. Neuropharmacol. 28, 38–49. [DOI] [PubMed] [Google Scholar]
- Niethammer M.; Ford B. (2007) Permanent lithium-induced cerebellar toxicity: three cases and review of the literature. Mov. Disord. 22, 570–573. [DOI] [PubMed] [Google Scholar]
- Tesio L.; Porta G. L.; Messa E. (1987) Cerebellar syndrome in lithium poisoning: a case of partial recovery. J. Neurol., Neurosurg. Psychiatry 50, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto A.; Koizumi N.; Itoh N.; Shigematsy H. (1993) An autopsy case of cerebellar degeneration following lithium intoxication with neuroleptic malignant syndrome. Acta Pathol. Jpn. 43, 55–58. [DOI] [PubMed] [Google Scholar]
- Dethy S.; Manto M.; Bastianelli E.; Gangji V.; Laute M. A.; Goldman S.; Hildebrand J. (1997) Cerebellar spongiform degeneration induced by acute lithium intoxication in the rat. Neurosci. Lett. 224, 25–28. [DOI] [PubMed] [Google Scholar]
- Dimitrova M.; Petrova E.; Gluhcheva Y.; Kadiysky D.; Dimitrova S.; Kolyovska V.; Deleva D. (2013) Neurodegenerative Changes in rat produced by lithium treatment. J. Toxicol Environ. Health, Part A 76, 304–310. [DOI] [PubMed] [Google Scholar]
- Bachmann R. F.; Wang Y.; Yuan P.; Zhou R.; Li X.; Alesci S.; Du J.; Manji H. K. (2009) Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int. J. Neuropsychopharmacol. 12, 805–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R.; Senatorov V.; Kanai H.; Leeds P.; Chuang D. M. (2003) Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience 117, 55–61. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Chang M. Y.; Yu I. T.; Kim J. H.; Lee S. H.; Lee Y. S.; Son H. (2004) Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J. Neurochem. 89, 324–336. [DOI] [PubMed] [Google Scholar]
- Young A. H.; Hammond J. M. (2007) Lithium in mood disorders: increasing evidence base, declineing use?. Br J. Psychiatry 191, 474–476. [DOI] [PubMed] [Google Scholar]
- Grandjean E. M.; Aubry J. M. (2009) Lithium: updated human knowledge using an evidence-based approach: part III: clinical safety. CNS Drugs 23, 397–418. [DOI] [PubMed] [Google Scholar]
- Aprahamian I.; Santos F. S.; Santos B.; Talib L.; Diniz B. S.; Radanovic M.; Gattaz W. F.; Forlenza O. V.. Long-term, low-dose lithium treatment does not impair renal function in the elderly: a two-year placebo-controlled trial followed by single-blind extension. J. Clin. Psychiatry 2014, in press [DOI] [PubMed]
- Diniz B. S.; Nunes P. V.; Yassuda M. S.; Pereira F. S.; Flaks M. K.; Viola L. F.; Radanovic M.; Abreu I. D.; Borelli D. T.; Gattaz W. F.; Forlenza O. V. (2008) Mild cognitive impairment: cognitive screening or neuropsychological assessment?. Rev. Bras. Psiquiatr. 30, 316–321. [DOI] [PubMed] [Google Scholar]
- Petersen R. C.; Smith G. E.; Waring S. C.; Ivnik R. J.; Tangalos E. G.; Kokmen E. (1999) Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. [DOI] [PubMed] [Google Scholar]
- Lancaster J. L.; Rainey L. H.; Summerlin J. L.; Freitas C. S.; Fox P. T.; Evans A. C.; Toga A. W.; Mazziotta J. C. (1997) Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward-transform method. Hum. Brain Mapp. 5, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J. L.; Woldorff M. G.; Parsons L. M.; Liotti M.; Freitas C. S.; Rainey L.; Kochunov P. V.; Nickerson D.; Mikiten S. A.; Fox P. T. (2000) Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 10, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]


