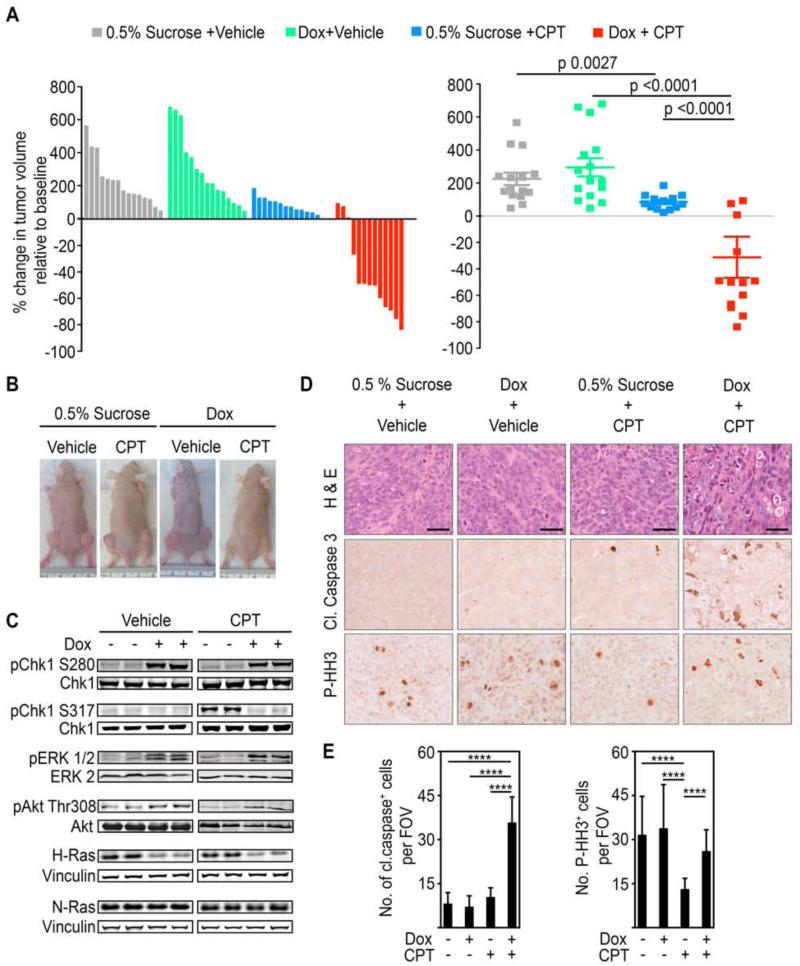
Figure 7. WT-H-Ras knockdown in established mutant K-Ras tumors impairs cell cycle arrest induced by DNA damaging chemotherapeutic agents and leads to tumor regression.
(A) Waterfall plot and scatter plot showing percentage change in the volume of subcutaneous DLD1 K-RasMut tumors in nude mice five days after the last dose of irinotecan (CPT) administration. Percentage change was determined relative to the tumor volume at the start of irinotecan (CPT) treatment for each individual tumor. Mice engrafted with 2×106 DLD1 K-RasMut cells stable for the doxycycline-inducible H-Ras 1 sh were given either doxycycline or vehicle-only (0.5% sucrose) as a control via their drinking water once tumors attained ~100 mm3. Irinotecan (CPT) administration (50 mg/kg every other day for 6 days (q2dx3)) was initiated when tumors reached ~250 mm3. Error bars, mean ± SEM.
(B) Representative xenograft tumors are shown.
(C) WCLs of tumor tissue obtained from the indicated animals 24 hr after the initiation of irinotecan (CPT) administration were immunoblotted with antibodies for the indicated proteins.
(D-E) Tumor sections from mice in (A) treated as indicated were stained for hematoxylin and eosin (H&E), anti-cleaved caspase 3, or anti-P-HH3 antibody. Representative images are shown in (D) and quantifications are shown in (E). Scale bars, 40 μm. Cleaved caspase 3 or P-HH3 positive cells were counted per field of view (FOV) at 20x magnification. Error bars, mean ± SD, n=3 mice per group, 4 FOV per mouse. ****p<0.0001. See also Figure S7.