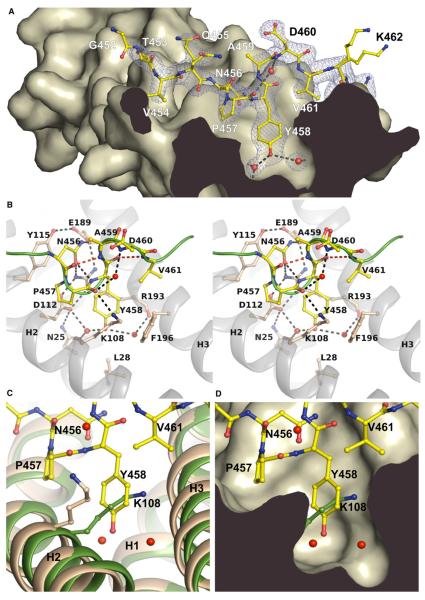
Figure 2. Atomic Details of the I-BARIRSp53:Tir Complex.
(A) Profile of the binding pocket. The Tir-derived peptide 452GTVQNPYADVKT463 is drawn in yellow with its electron density map in blue. Dashed lines indicate hydrogen bonds. Tyr458 of the NPY motif is centered by two water molecules (red) in the interior of the binding pocket.
(B) Stereo view of the interactions of the Tir-derived peptide comprising the NPY motif (456–458). Residues involved in the complex interface and in β-turn formation are labeled. The green line represents the backbone of the peptide. Hydrogen bonds involved in β-turn formation are shown as red dashed lines (see also Movie S1). (C and D) Superposition of the free I-BAR structure of Millard et al. (2005) (green, PDB 1Y2O) and the peptide-bound domain (wheat). The side chain of Lys108 blocks the binding pocket in the free structure. The conformation of the side chain of Lys108 in the free I-BAR structure of Suetsugu et al. (2006) is similar to the complex (1WDZ, not shown).
See also Movie S1.