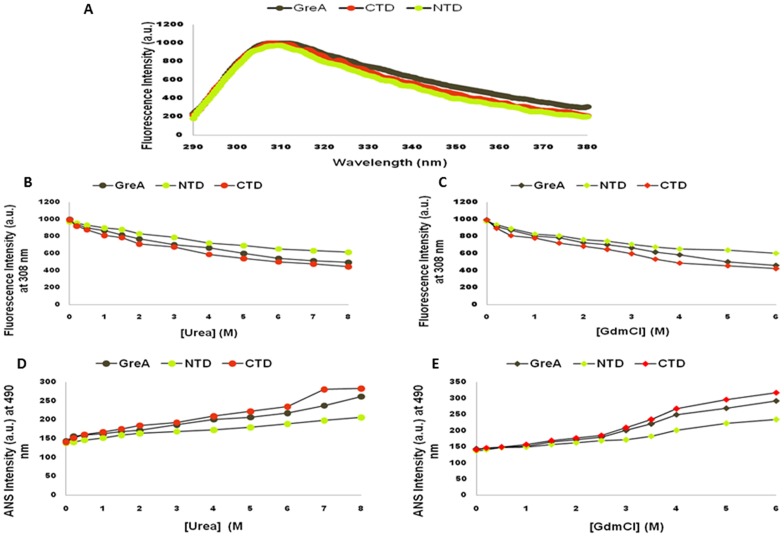
Figure 7. Intrinsic fluorescence emission spectra and ANS binding study of Wol GreA with its two domains.
(A) To explore the intrinsic fluorescence emission of buried Tyr residues that subsist in Wol GreA, Wol NTD, and Wol CTD, the respective spectra were recorded in the range of 290–380-nm on excitation at 275-nm. Maximum peaks were generated at 308-nm which is the characteristic peak of buried Tyr indicating that Wol GreA and its domains exist in the properly folded native conformation. (B) Effect of different conc. of urea from 0.1 M to 8 M on the fluorescence intensity of Wol GreA (grey sphere), Wol NTD (green sphere), and Wol CTD (red sphere) at 308 nm under neutral pH 7.0. (C) Effect of different conc. of GdmCl ranging between 0.1-M and 6-M on the fluorescence intensity of Wol GreA (grey diamond), Wol NTD (green diamond), and Wol CTD (red diamond) at 308-nm under neutral pH 7.0. (D) Binding of ANS with exposed hydrophobic pockets of Wol GreA (grey sphere), Wol NTD (green sphere), and Wol CTD (red sphere) generated at various conc. of urea. Protein to ANS ratio of 1∶2 in stoichiometry was used and the spectra were recorded in the range of 400–550-nm on excitation at 390-nm at 25°C under neutral conditions and the intensity graph of ANS was plotted at 490-nm. (E) Binding of ANS with exposed hydrophobic pockets of wild (grey diamond), Wol NTD (green diamond), and Wol CTD (red diamond) was observed at various conc. of GdmCl. Protein to ANS stoichiometry remained the same as in urea and the spectra were recorded in the region of 400–550-nm on excitation at 390-nm at 25°C under similar conditions, and finally, the intensity graph of ANS was plotted at 490-nm.