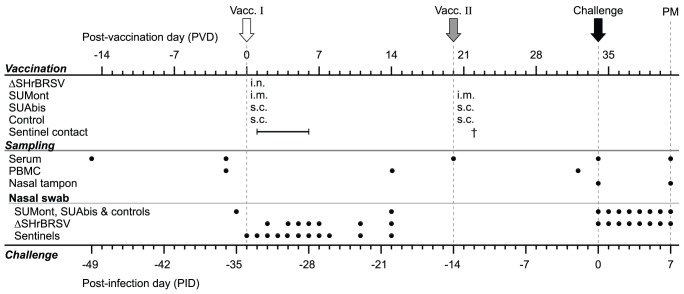
Figure 1. Experiment timeline, vaccination and sampling.
Twenty calves with moderate titers of BRSV-specific serum antibodies (MDA) were allocated into 4 groups and vaccinated as indicated in the figure; all were vaccinated on post-vaccination day (PVD) 0 (Vacc. I, white arrow) with either (a) 5×106 pfu of ΔSHrBRSV intranasally (i.n.); (b) BRSV and HRSV recombinant protein subunits (SU) adjuvanted by Montanide (SUMont) intramuscularly (i.m.), (c) SU adjuvanted by AbISCO-300 (SUAbis) subcutaneously (s.c.), or (d) adjuvant alone s.c. (Controls). On PVD 20, all animals except those immunized with ΔSHrBRSV, were boosted with the same formulation and route as for Vacc. I (Vacc. II, gray arrow). Three BRSV-seronegative calves were housed in contact with ΔSHrBRSV-infected animals to determine transmission of the vaccine virus (Sentinel calves), and monitored until euthanized (†) on PVD 22. On PVD 20, one calf in group c was euthanized due to traumatic injury. On post-infection day (PID) 0, all calves were challenged i.n. with 104 pfu virulent BRSV (black arrow), and clinically scored daily until PID 7. Throughout the experiment, samples were collected, as indicated in the figure, to analyze antibodies in serum and nasal secretions, ex-vivo response of peripheral blood mononuclear cells (PBMC) to restimulation with BRSV, and virus shedding in nasal secretions (Nasal swab). At post-mortem examination (PM), lung lesions were recorded and tissue samples collected, as well as bronchoalveolar lavage (BAL) samples for antibody, BRSV RT-PCR and virus isolation.