Abstract
Jak2, a member of the Janus kinase family of non-receptor protein tyrosine kinases, is activated in response to a variety of cytokines, and functions in survival and proliferation of cells. An activating JAK2V617F mutation has been found in most patients with myeloproliferative neoplasms, and patients treated with Jak2 inhibitors show significant hematopoietic toxicities. However, the role of Jak2 in adult hematopoietic stem cells (HSCs) has not been clearly elucidated. Using a conditional Jak2 knockout allele, we have found that Jak2 deletion results in rapid loss of HSCs/progenitors leading to bone marrow failure and early lethality in adult mice. Jak2 deficiency causes marked impairment in HSC function, and the mutant HSCs are severely defective in reconstituting hematopoiesis in recipient animals. Jak2 deficiency also causes significant apoptosis and loss of quiescence in HSC-enriched LSK (Lin−Sca-1+c-kit+) cells. Jak2-deficient LSK cells exhibit elevated reactive oxygen species levels and enhanced p38 MAPK activation. Mutant LSK cells also show defective Stat5, Erk and Akt activation in response to thrombopoietin and stem cell factor. Gene expression analysis reveals significant downregulation of genes related to HSC quiescence and self-renewal in Jak2-deficient LSK cells. These data suggest that Jak2 plays a critical role in the maintenance and function of adult HSCs.
INTRODUCTION
Hematopoietic stem cells (HSCs) play an essential role in hematopoiesis through their unique ability to self-renew and differentiate into progenitors of all types of mature blood cells. A majority of HSCs are maintained in a state of quiescence to prevent HSC exhaustion and support long-term hematopoiesis. Understanding the mechanisms by which quiescence, survival, self-renewal and differentiation of HSCs are regulated is critical for rational design of therapies for blood disorders.
Janus kinase 2 (Jak2) is a ubiquitously expressed non-receptor protein tyrosine kinase which is activated in response to various growth factors and cytokines [1,2]. Germ-line deletion of Jak2 causes impairment of fetal liver erythropoiesis leading to embryonic lethality in mice [3,4]. Deletion of Jak2 at post-natal or adult stage results in anemia and thrombocytopenia in mice [5] suggesting a role for Jak2 in erythroid/megakaryocytic development. However, the role of Jak2 in the maintenance and function of adult HSCs has not been clearly elucidated. Also, the mechanism by which Jak2 regulates HSC function remains unknown.
An activating JAK2V617F mutation has been associated with most cases of myeloproliferative neoplasms (MPNs) [6–10]. MPNs are considered to be clonal stem cell-derived disorders, which are characterized by excessive production of myeloid/erythroid/megakarocytic lineage cells [11,12]. Several Jak2 inhibitors have been developed for treatment of MPNs, but most patients treated with Jak2 inhibitors exhibit significant hematopoietic toxicities [13–15]. Therefore, understanding the role of Jak2 in adult HSCs/progenitors is of considerable significance and has potential clinical implications.
In this report, we examined the role of Jak2 in adult HSCs/progenitors using conditional Jak2 knockout and MxCre mice. We found that Jak2-deficiency causes loss of quiescence, increased apoptosis and profound defects in HSC function resulting in early deaths in adult mice. We also observe that Jak2 is cell autonomously required for HSC self-renewal. Jak2-deficiency causes impairment of Stat5, Erk and Akt signaling mediated by thrombopoietin (TPO) and stem cell factor (SCF) in HSC-enriched LSK cells. Gene expression analyses also reveal significant downregulation of HSC-related gene sets in Jak2-deficient LSK cells. Together, these results suggest an essential role for Jak2 in the maintenance and function of adult HSCs.
MATERIALS AND METHODS
Mice
Conditional Jak2 floxed (Jak2fl/fl) [16] mice were crossed with MxCre [17] mice to generate MxCre;Jak2fl/fl mice. Cre expression was induced by intraperitoneal injection of 5 doses of 300 μg of polyinosine-polycytosine (pI; pC, GE Healthcare). C57BL6/J (CD45.2) and BL6.SJL-Ptprca Pep3b/BoyJ (CD45.1) mice were purchased from the Jackson laboratory. All animal studies were approved by the Institutional Animal Care and Use Committee of SUNY Upstate Medical University.
Blood and tissue analysis
Peripheral blood cell counts were determined using Hemavet 950FS (Drew Scientific). Blood smears were stained with Wright-Giemsa. For histopathologic analysis, mouse tissue specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin.
Flow cytometry
Single cell-suspensions were prepared from BM and red cells were lysed with red cell lysis solution. Cells were washed and resuspended in PBS plus 2% FBS. For HSC/progenitor analysis, BM cells were stained for 1 hour on ice with antibodies against c-Kit, Sca-1, CD34, CD16/32 (FcγR II/III), CD41, CD48, CD150, CD135, and antibodies against lineage (Lin) markers including CD3, CD4, CD8, CD19, B220, Gr-1, CD127, and Ter119. Flow cytometry antibodies were purchased from eBioscience, BioLegend or BD Biosciences. BrdU incorporation in LSK was determined using the FITC BrdU Flow Kit (BD Biosciences) according to the manufacturer’s protocol. For Hoechst 33342 (HO; Sigma-Aldrich) and Pyronin Y (PY; Sigma-Aldrich) staining, Lin− cells were first enriched by magnetic activated cell sorting (MACS; Miltenyi) and then stained with LSK surface markers along with HO/PY in the presence of verapamil (Sigma-Aldrich). To assess apoptosis, BM cells were stained with LSK surface markers and Annexin V followed by flow cytometric analysis. To assess signaling in LSK cells, MACS-enriched Lin− BM cells were starved for 1 hour before stimulation with stem cell factor (SCF; 50 ng/ml) or thrombopoietin (TPO; 50 ng/ml) for 5 or 15 minutes. Cells were fixed with 4% paraformaldehyde, permeabilized with acetone, and then stained with LSK surface makers along with Alexa488-conjugated phospho-specific antibodies (Cell Signaling Technology). Flow cytometry was performed with an LSRII (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Colony-forming assays
BM (2 × 104) cells were plated in duplicates in complete methylcellulose medium (MethoCult M3434; StemCell Technologies, Vancouver, Canada). Burst-forming units-erythroid (BFU-E), granulocyte-macrophage colony-forming units (CFU-GM), and colony-forming units-granulocyte, erythrocyte macrophage, megakaryocyte (CFU-GEMM) colonies were scored on day 7. To detect colony-forming units-erythroid (CFU-E) colonies, BM cells were plated in MethoCult M3234 medium (StemCell Technologies) in the presence of erythropoietin (Epo). CFU-E colonies were counted after 2 days by staining with benzidine solution (Sigma-Aldrich). To detect colony-forming units-megakaryocyte (CFU-Mk), BM cells were plated in collagen-based MegaCult medium (StemCell Technologies) in the presence of IL-6, IL-11, and Tpo. CFU-Mk colonies were scored after 7–8 days according to manufacturer’s protocol.
BM transplantation assays
For non-competitive BM transplantation (BMT) assay, BM cells from pI;pC induced MxCre;Jak2fl/fl or littermate control (Jak2fl/fl) mice (CD45.2+) were transplanted into lethally irradiated CD45.1+ recipient mice by retro-orbital injection without competitor. For competitive reconstitution assay, BM cells from control (Jak2fl/fl) or MxCre;Jak2fl/fl mice (CD45.2+) were mixed with CD45.1+ competitor BM cells at 1:1 or 10:1 (CD45.2:CD45.1) ratio and transplanted into lethally irradiated CD45.1+ recipients by retro-orbital injection. In some cases, Jak2 was deleted after the BMT in hematopoietic compartments of recipient animals by injection with 5 doses of pI;pC at 4 weeks after transplantation. Chimerism was measured in peripheral blood and BM of transplanted animals by CD45.2 and CD45.1 expression.
Microarray analysis
LSK cells were sorted from the BM of control (Jak2fl/fl) or MxCre;Jak2fl/fl mice 4 weeks after pI;pC injection. Total RNA was extracted from the LSK cells using Qiagen RNeasy Mini kit (Qiagen). RNA samples were processed using the Ovation Pico WTA System V2 (NuGEN Technologies), and biotin labeled with the Encore Biotin Module (NuGEN Technologies). Microarry experiments were performed in the SUNY Upstate Medical University Microarray Core Facility using the GeneChip Mouse Gene 1.0 ST Array (Affymetrix). Arrays were scanned with the Affymetrix GeneChip Scanner 7G Plus. The threshold for considering genes differentially expressed was 1.4-fold difference with a P value <0.05. Heat maps were generated using MultiExperiment Viewer software available from (http://www.tm4.org/mev.html). Gene set enrichment analysis [18] was performed with GSEA v2.08 software available from the Broad Institute (http://www.broad.mit.edu/gsea). Microarray data were also analyzed with Ingenuity Pathway Analysis (Ingenuity Systems).
Quantitative real-time PCR
Total RNA was extracted from sorted LSK cells obtained from control (Jak2fl/fl) or MxCre;Jak2fl/fl mice by RNeasy Mini Kit (Qiagen) and reverse transcribed by using QuantiTect Reverse Transcription kit (Qiagen). Quantitative real-time PCR was performed using SYBR Green PCR master mix (Roche Diagnostics). The data were normalized to 18S and fold-change determined by the ΔΔCt method.
Statistical Analysis
Results are expressed as mean ± SEM, and data were analyzed by the 2-tailed Student t test. A value of P less than 0.05 was considered to be statistically significant.
RESULTS
Deletion of Jak2 results in fatal BM failure in adult mice
To assess the function of Jak2 in the adult hematopoietic system, we crossed Jak2 floxed (Jak2fl/fl) mice with MxCre mice which express Cre recombinase in all hematopoietic compartments including HSCs in response to interferon or polyinosine-polycytosine (pI;pC) treatment [17]. We conditionally deleted Jak2 in MxCre;Jak2fl/fl mice by administration of 5 doses of pI;pC at 4 weeks after birth. As shown in Fig. 1A, Jak2 was almost completely deleted in the bone marrow (BM) of MxCre;Jak2fl/fl mice upon pI;pC induction. All induced MxCre;Jak2fl/fl (hereafter Jak2-deficient) mice became moribund or died between 25 and 44 days with the mean survival of 36 days after first pI:pC injection (Fig. 1B). Therefore, all analyses were performed at 4 weeks after pI:pC induction unless otherwise specified. Mice with homozygous Jak2 deletion exhibited significantly reduced numbers of white blood cell (WBC), neutrophil, red blood cell (RBC), hematocrit and platelet counts in their peripheral blood compared with control animals (Supplementary Fig. S1). Mice with heterozygous Jak2 deletion, however, did not exhibit any significant decrease in their peripheral blood counts compared with control animals (Supplementary Fig. S1). H&E staining of the BM sections from Jak2-deficient animals showed severe BM aplasia (Fig. 1C). Together, these data suggest that deletion of Jak2 results in severe defects in the hematopoietic compartment.
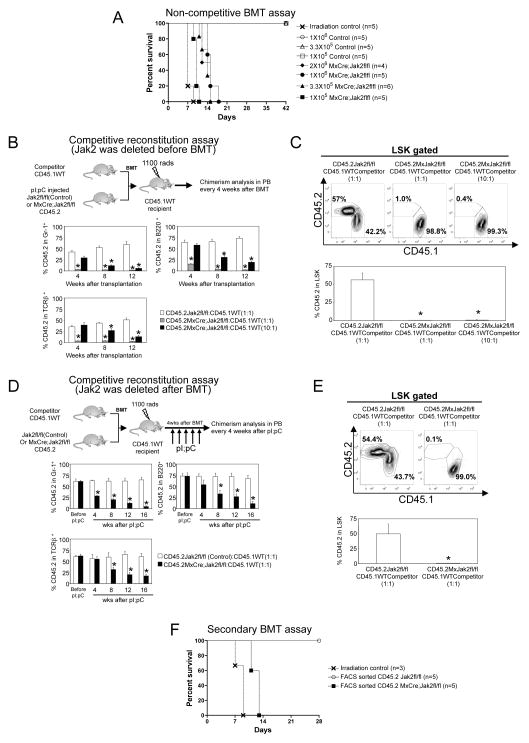
Figure 1. Deletion of Jak2 leads to fatal BM failure and loss of HSCs/progenitors.
(A) The expression of Jak2 in the BM of Jak2fl/fl (control) and MxCre;Jak2fl/fl (Jak2-deficient) mice was determined by immunoblotting at 4 weeks after pI;pC injection. Erk2 was used as a loading control. (B) Kaplan-Meier survival analysis of MxCre;Jak2fl/fl (n=24) and littermate control (Jak2fl/fl) (n=24) mice after pI;pC treatment. MxCre;Jak2fl/fl mice have a median survival of 36 days after 1st pI;pC injection; *P< 0.05, log-rank test. (C) H&E staining of the BM sections (200×) demonstrates pancytopenia and severe aplasia in Jak2-deficient BM. (D) Flow cytometric analysis of LSK compartment (Lin−Sca1+c-kit+) and myeloid progenitors including CMP (Lin−Sca1+c-kit+CD34+FcγRII/IIIlo), CLP (Lin−CD127+Sca1+c-kit+), GMP (Lin−Sca1+c-kit+CD34+FcγRII/IIIhi), and MEP (Lin−Sca1+c-kit+CD34−FcγRII/III−) in the BM from control or Jak2-deficient (MxCre;Jak2fl/fl) mice (n=10) at 4 weeks after pI:pC injection. The percentages and absolute numbers of LSK, CLP, CMP, GMP, and MEP are shown in bar graphs as mean ± SEM. *P< 0.05. (E) Flow cytometric analysis of LT-HSC (Lin−Sca1+c-kit+CD34−CD135−), ST-HSC (Lin−Sca1+c-kit+CD34+CD135−), and MPP (Lin−Sca1+c-kit+CD34+CD135+) in the BM from control or Jak2-deficient (MxCre;Jak2fl/fl) mice at 4 weeks after pI:pC injection (n=7). Representative contour plots are shown in the top. The percentages and absolute numbers of LT-HSC, ST-HSC, and MPP are shown in bar graphs as mean ± SEM. *P< 0.05. (F) Flow cytometric analysis of SLAM-HSC (Lin−Sca1+c-kit+CD150+CD41−CD48−) in the BM from control or Jak2-deficient (MxCre;Jak2fl/fl) mice at 4 weeks after pI:pC injection (n=5). Representative contour plots are shown on the top. The percentages and absolute numbers of SLAM-HSC are shown in bar graphs as mean ± SEM; *P< 0.05. (G, H) Hematopoietic progenitor colony assays. Total BM cells (2×104) (G) or sorted LSK cells (100) (H) from control or Jak2-deficient (MxCre;Jak2fl/fl) mice were plated in methylcellulose medium MethoCult 3434 (StemCell Technologies) with cytokines. BFU-E, CFU-GM and CFU-GEMM colonies were scored 7–8 days after plating. To assess CFU-E colonies, BM cells were plated in methycellulose medium in the presence of Epo. CFU-E colonies were scored 3 days after plating. To assess CFU-Mk colonies, BM cells were plated in collagen-based MegaCult media with IL-6, IL-11, and Tpo. Colonies were counted after 7–8 days. Note that deletion of Jak2 significantly reduces hematopoietic progenitor colonies in the total BM or sorted LSK cells from MxCre;Jak2fl/fl mice (n=5). *P< 0.05. (I) Cell autonomous BMT assay. BM cells (1×106 cells) from uninduced MxCre;Jak2fl/fl or littermate control (Jak2fl/fl) mice (CD45.2+) were transplanted into lethally irradiated CD45.1 recipients. Deletion of Jak2 was induced into the donor-derived hematopoietic cells of recipient animals by injecting pI;pC four weeks after BMT. Note that donor-derived (CD45.2+) HSC (LSKCD150+CD41−CD48−) was significantly reduced in recipients of Jak2-deficient BM (n=5, *P< 0.05). (J) BM cells (1×106 cells) from CD45.1 WT mice were transplanted into lethally-irradiated CD45.2 control (Jak2fl/fl) or MxCre;Jak2fl/fl recipients. Four weeks after BMT to allow establishment of steady-state hematopoiesis, Jak2 was deleted from non-hematopoietic cells in the mutant recipients with 5 doses of pI;pC injection. Note that CD45.1+ WT donor-derived HSCs (LSKCD150+CD41−CD48−) were not reduced upon induction of Jak2 deletion in mutant recipients (n=3).
The fatal BM failure in Jak2-deficient mice prompted us to examine the HSC/progenitor compartments in the BM of these animals. We observed a marked decrease in the frequencies and absolute numbers of HSC-enriched Lin−Sca-1+c-kit+ (LSK) and early progenitors including common lymphoid progenitors (CLP), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP), and megakaryocyte-erythroid progenitors (MEP) in the BM of Jak2-deficient mice (Fig. 1D). Further analysis of the HSC compartment revealed a marked reduction in the number of LT-HSC (defined as Lin−Sca1+c-kit+CD34−CD135− or Lin−Sca1+c-kit+CD150+CD41−CD48−), ST-HSC (Lin−Sca1+c-kit+CD34+CD135−), and MPP (Lin−Sca1+c-kit+CD34+CD135+) [19,20] in Jak2-deficient BM (Fig. 1E, 1F), indicating a defect at the earliest stage of adult hematopoietic development.
Next, we performed colony-forming unit (CFU) assays to assess HSC/progenitor function. Consistent with the flow cytometric data, Jak2-deficient BM cells produced markedly reduced numbers of CFU-E, BFU-E, CFU-GM, CFU-GEMM and CFU-Mk colonies compared with control BM cells (Fig. 1G). Similar results were obtained when sorted LSK cells were used in colony assays (Fig. 1H). Thus, loss of Jak2 results in decrease in the number and differentiation potential of the HSCs/progenitors.
To determine whether the loss of HSCs in Jak2-deficient animals is cell autonomous, BM cells from uninduced MxCre;Jak2fl/fl mice were transplanted into lethally irradiated congenic CD45.1+ WT animals. Four weeks after transplantation, recipients were injected with pI:pC, and the percentages of donor-derived (CD45.2+) HSCs were assessed at 8 weeks after pI:pC induction using flow cytometry. As shown in Fig. 1I, the number of donor-derived LT-HSCs was significantly reduced in recipient mice transplanted with MxCre;Jak2fl/fl BM compared with control BM, similar to those observed in primary Jak2-deficient animals. Furthermore, transplantation of WT CD45.1+ BM into MxCre;Jak2fl/fl (CD45.2+) recipient mice showed that donor-derived HSCs were not reduced upon induction of Jak2 deletion in the mutant recipients (Fig. 1J), indicating that deletion of Jak2 from a nonhematopoietic compartment does not contribute to the HSC phenotype. These data strongly suggest that the reduction of HSCs in Jak2-deficient mice is cell autonomous.
Jak2 deficiency impairs long-term engraftment and self-renewing capacity of adult HSCs
To examine if Jak2-deficient HSCs are intrinsically capable of engraftment and/or differentiation, different doses of BM cells from control or Jak2-deficient mice were transplanted into lethally irradiated recipients in the absence of competitor cells. Whereas control BM cells (1 × 105) were able to efficiently engraft, Jak2-deficient BM cells failed to engraft even at cell doses 20 times higher than controls (2 × 106 total BM cells) and all recipients of Jak2-deficient BM died within 18 days after transplantation (Fig. 2A).
Figure 2. Defective stem cell function in Jak2-deficient mice.
(A) Non-competitive BMT assay. Different doses of BM cells from control (1×105–1×106 cells) or MxCre;Jak2fl/fl (1×105–2×106 cells) mice were transplanted into lethally irradiated CD45.1 recipient mice in absence of competitor cells. Note that Jak2-deficient BM cells failed to reconstitute hematopoiesis in recipient animals even when transplanted 20-times more mutant BM cells than control BM cells. (B) Competitive reconstitution assay with Jak2 deletion before BMT. Total BM cells from pI;pC induced control or MxCre;Jak2fl/fl (CD45.2) mice were transplanted into lethally irradiated CD45.1 recipient mice in competition with BM from CD45.1 WT mice, at different ratios (1:1 or 10:1) of donor versus competitor. The percentages of donor derived (CD45.2+) myeloid cells (Gr-1+), B cells (B220+), and T cells (TCRβ+) in peripheral blood at different time points after transplantation are shown as mean ± SEM. (n=5, *P< 0.05). (C) The percentages of donor-derived (CD45.2+) LSK in the BM of the recipient animals are shown at 12 weeks after transplantation (n=5). (D) Competitive reconstitution assay with Jak2 deletion after BMT. Total BM cells from uninduced MxCre;Jak2fl/fl or littermate control (CD45.2) mice were transplanted into lethally irradiated CD45.1 recipient mice in competition with BM from CD45.1 WT mice at 1:1 ratio. Four weeks after BMT, recipient mice were treated with 5 doses of pI;pC to induce Jak2 deletion after hematopoietic reconstitution. The percentages of donor derived (CD45.2+) myeloid cells (Gr-1+), B cells (B220+), and T cells (TCRβ+) in peripheral blood was measured every four weeks after pI;pC injection. (E) The percentages of donor-derived (CD45.2+) LSK in the BM of the recipient animals at 20 weeks after transplantation (16 weeks after pI;pC injection) are shown as mean ± SEM. (n=5, *P< 0.05). (F) Survival curves of secondary transplanted animals. CD45.2+ (donor-derived) cells from primary transplanted mice (as in Fig. 2D) were FACS sorted 12 weeks after pI;pC injection, and injected into lethally irradiated CD45.1 secondary recipient mice (5×105 CD45.2+ cells/recipient) (n=5). Note that Jak2-deficient cells failed to engraft secondary recipients and all secondary recipients that had received Jak2-deficient cells died within 2 weeks after transplantation.
We next performed competitive repopulation assays to further evaluate the function of Jak2-deficient HSCs in hematopoietic reconstitution. BM cells from pI;pC induced control or Jak2-deficient mice (CD45.2+) were mixed with CD45.1+ competitor BM cells at a ratio of 1:1 or 10:1 and then transplanted into lethally irradiated congenic recipient animals (CD45.1+). Chimerism analysis in peripheral blood (PB) of transplanted animals revealed that BM cells from Jak2-deficient mice were completely unable to compete with WT BM cells even when transplanted at a 10-fold higher dose of mutant (Jak2-deficient) cells than competitor cells (Fig. 2B). The percentages of mutant (Jak2-deficient) donor-derived (CD45.2+) myeloid, B and T cells were significantly lower than those derived from control (Jak2fl/fl) BM donor (Fig. 2B). At 12 weeks after transplantation, mutant donor-derived (CD45.2+) LSK cells were almost undetectable in the BM of recipient animals, whereas more than 50% LSK cells in recipients that had received control BM were donor-derived (CD45.2+) (Fig. 2C). These data suggest that Jak2-deficient HSCs are functionally defective in hematopoietic reconstitution.
To exclude the possibility that the functional defects observed in Jak2-deficient HSCs is not only due to poor engraftment of Jak2-deficient HSCs, we also performed competitive repopulation assays using uninduced BM cells from MxCre;Jak2fl/fl or control (Jak2fl/fl) mice (CD45.2+) along with CD45.1+ WT competitor BM cells at a ratio of 1:1 and transplanted into lethally irradiated congenic CD45.1 animals. Four weeks after transplantation (to allow establishment of steady-state hematopoiesis), recipients were injected with 5 doses of pI;pC. All mice receiving control (Jak2fl/fl) or MxCre;Jak2fl/fl BM showed comparable levels of CD45.2 engraftment in the myeloid, B and T cells before pI;pC injection (Fig. 2D). Whereas recipients of control (Jak2fl/fl) BM maintained more than 50% CD45.2+ myeloid, B and T cells in their peripheral blood for 16 weeks after pI;pC treatment, the percentage of CD45.2+ cells in the peripheral blood was progressively and significantly reduced after pI;pC induction in mice that had received MxCre;Jak2fl/fl BM (Fig. 2D). At 16 weeks after pI;pC induction, MxCre;Jak2fl/fl BM-derived (CD45.2+) LSK cells were markedly diminished in the recipient animals, whereas ~50% LSK cells in recipients the had received control (Jak2fl/fl) BM were CD45.2+ (Fig. 2E). These data strongly suggest that Jak2 deficiency leads to severe functional impairment in stem cells.
Since deletion of Jak2 in the BM leads to exhaustion of stem cells and profound defects in hematopoietic reconstitution in the primary transplant recipients (Fig. 2A, 2C and 2E), we examined whether Jak2 is required for HSC self-renewal using a secondary transplantation experiment. Jak2 deletion in primary mice (before the BMT) severely impaired hematopoietic reconstitution (Fig 2A and 2B) and thus cannot be utilized for secondary transplantation experiment. So, we utilized the BM from the recipient animals in which Jak2 was deleted from hematopoietic compartments by pI;pC induction after the hematopoietic reconstitution (as in Fig 2D). BM cells from uninduced control (Jak2fl/fl) or MxCre;Jak2fl/fl (CD45.2+) cells were transplanted into CD45.1 recipients along with competitor (CD45.1+) cells. Four weeks after BMT, pI;pC was injected to induce Jak2 deletion in hematopoietic compartments of the recipient animals (as in Fig 2D). At 12 weeks after pI;pC injection, BM cells were harvested and sorted for the donor CD45.2 marker. Sorted CD45.2+ (donor-derived) cells were then transplanted (5×105 cells/recipient) into lethally irradiated secondary recipients (CD45.1) without the competitor cells. Whereas CD45.2+ control cells were able to efficiently engraft and reconstitute hematopoiesis in secondary recipients, Jak2-deficient cells were unable to engraft secondary recipients and all mice receiving CD45.2+ Jak2-deficient cells died within 14 days after transplantation (Fig. 2F). These data suggest that Jak2 is absolutely required for self-renewal of adult HSCs.
Jak2 is required for the maintenance of quiescence and survival of LSK cells
Most HSCs remain in the quiescent state (G0) for long-term maintenance of the HSC pool in adult BM. Reduced numbers of HSCs and progenitors in Jak2-deficient mice could be due to loss of quiescence and/or increased cell death. To determine if Jak2 is required for the maintenance of HSC quiescence, we assessed the cell cycle distribution of HSC-enriched LSK cells in control and Jak2-deficient mice at 4 weeks after pI;pC induction using BrdU incorporation assay. BrdU was injected into control and Jak2-deficient mice 4 hours prior to BM harvest. We observed significantly more BrdU incorporation in Jak2-deficient LSK with a much higher proportion in the S phase and lower proportion in the G0/G1 phase compared with control LSK (Fig. 3A). Further analysis of the cell cycle by Hoechst 33342/Pyronin Y staining revealed that significantly fewer number of Jak2-deficient LSK cells were in the quiescent G0 phase, with a higher proportion in S/G2/M (Fig. 3B). Notably, deletion of Jak2 did not induce proliferation or any significant change in cell cycle within myeloid progenitors (Lin− c-kit+) (Supplemental Fig. S2). These results suggest that Jak2 deficiency results in HSC-specific loss of quiescence, which may contribute to rapid exhaustion of HSCs in Jak2-deficient animals.
Figure 3. Reduced quiescence and increased apoptosis in Jak2-deficient LSK cells.

(A) Control or Jak2-deficient mice were injected with BrdU and sacrificed 4 hours later. Representative FACS plots (in the top panel) illustrate BrdU/DAPI staining in LSK cells. Bar graphs in the bottom panel show percentages of BrdU+ LSK cells in G0/G1, S and G2/M. Data from five independent experiments are shown as mean ± SEM. *P< 0.05. (B) Flow cytometric analysis of Hoechst and Pyronin Y staining on LSK cells from control and Jak2-deficient mice BM. Representative contour plots are shown on the top panel. Bar graphs in the bottom panel show percentages of LSK cells in G0, G1 and S/G2/M. Data from five independent experiments are shown as mean ± SEM. *P< 0.05. (C) Apoptosis in LSK cells from control or Jak2-deficient mice was determined using Annexin V and DAPI staining. Representative contour plots are shown on the top. Bar graphs (in the bottom) represent percentages of apoptotic LSK cells in control or Jak2-deficient mice. Data from five independent experiments are shown as mean ± SEM. *P< 0.05.
To assess if Jak2 is required for survival of HSCs, we performed annexin V staining of LSK cells from control and Jak2-deficient mice. Annexin V staining showed significantly (~ 9 fold) increased apoptosis in Jak2-deficient LSK compared with control LSK (Fig. 3C), suggesting that Jak2 also plays an important role in survival of HSC pool. Collectively, our data indicate that reduced quiescence and increased apoptosis may account for loss of HSCs in Jak2-deficient mice.
Increased reactive oxygen species and p38 MAPK activation in Jak2-deficient LSK cells
It has been shown that elevated reactive oxygen species (ROS) promotes loss of quiescence and limits the lifespan of HSCs through p38 MAPK activation [21]. Therefore, we assessed whether the defects in HSC maintenance in Jak2-deficient mice were associated with increased levels of ROS and p38 MAPK activation. Intracellular concentration of ROS was measured in control and Jak2-deficient LSK cells by chloromethyl-2′-7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) staining. We observed a significant increase in ROS levels in Jak2-deficient LSK cells compared with control LSK cells (Fig. 4A). Activation of p38 MAPK was assessed in LSK cells by flow cytometry following intracellular staining with Alexa488-conjugated phospho-p38 MAPK antibody. We observed significantly (~ 3 fold) increased p38 MAPK phosphorylation in Jak2-deficient LSK cells compared with control LSK cells (Fig. 4B).
Figure 4. Elevated ROS levels and increased p38 MAPK activation in Jak2-deficient LSK cells.
(A) LSK cells from control (Jak2fl/fl) and Jak2-deficient mice were stained with CM-H2DCFDA to measure intracellular ROS. Jak2-deficient LSK cells showed increased levels of ROS compared with control LSK. Representative FACS plots are shown on the left. Mean values ± SEM are shown on the right (n=3, *P< 0.05). (B) Flow cytometric analysis for phospho-p38 MAPK in LSK cells. MACS-enriched Lin- cells from control or Jak2-deficient mice BM were starved for 1 hour in serum-free media at 37°C. Cells were fixed, permeabilized, and stained with appropriately conjugated antibodies against LSK surface markers and Alexa488 conjugated phospho-p38 MAPK. Representative FACS plots on intracellular phospho-p38 MAPK staining in LSK cells are shown on the left. The fold change in mean fluorescent intensity (MFI) of phospho-p38 MAPK in Jak2-deficient LSK is shown in bar graphs (on the right) as mean ± SEM (n=4, *P< 0.05). (C) NAC treatment of Jak2-deficient mice BM partially rescued the defects in hematopoietic reconstitution. Lin− BM cells from control or Jak2-deficient mice (CD45.2+) were treated with PBS or NAC ex vivo for 48 hours in StemSpan medium (Stem Cell Technologies) containing SCF (50 ng/ml) and Tpo (50 ng/ml) and then 1×106 cells were transplanted in the presence of 2×105 helper BM cells (CD45.1+) into lethally irradiated CD45.1 recipient mice. The percentages of donor-derived (CD45.2+) LSK in the BM of recipient animals were determined at 4 weeks after transplantation by flow cytometry. Representative contour plots are shown on the top panel. Bar graphs (in the bottom) show percentages of donor-derived (CD45.2+) LSK cells in recipient mice that had received PBS-treated or NAC-treated Jak2-deficient BM or control BM (n=4 in each group, *P< 0.05).
Next, we asked if reduction of ROS levels by treatment with ROS scavenger N-acetyl L-cysteine (NAC) could improve HSC function and prolong the lifespan of Jak2-deficient HSCs. We observed that ex vivo treatment with NAC partially, albeit significantly, rescued the hematopoietic reconstitution defects in Jak2-deficient HSCs (Fig. 4C). Whereas <1% donor-derived (CD45.2+) LSK cells were detected in the BM of recipient animals (CD45.1) that had received PBS-treated Jak2-deficient BM at 4 weeks after transplantation, ~20% LSK cells in recipients that had received NAC-treated Jak2-deficient BM were CD45.2+ (Fig. 4C). Together, these data suggest that Jak2-deficiency leads to elevated ROS levels, which may contribute to impairment in HSC maintenance.
Perturbed TPO and SCF signaling in Jak2-deficient LSK cells
TPO and SCF are required for HSC maintenance in vivo [22–26], and Jak2 has been shown to interact with TPO receptor Mpl and SCF receptor Kit [27,28]. Therefore, we investigated the effects of Jak2-deficiency in signaling mediated by TPO and SCF in HSC-enriched LSK cells. Intracellular phospho flow cytometric analysis revealed that TPO-evoked Stat5, Erk and Akt activation was markedly reduced in Jak2-deficient LSK cells compared with control LSK cells (Fig. 5A). Notably, control and Jak2-deficient LSK cells express similar levels of TPO receptor Mpl (Fig. 5B). Although SCF stimulation did not cause significant activation of Stat5 in either control or Jak2-deficient LSK cells, SCF-evoked Erk and Akt activation was substantially reduced in Jak2-deficient LSK cells compared with control LSK cells (Fig. 5C). Unlike Mpl, Kit expression was significantly reduced in Jak2-deficient lin−Sca-1+CD41−CD48−CD150+ cells (Fig. 5D), indicating that Jak2 may regulate Kit expression. Thus, the HSC phenotype in Jak2-deficient mice can be attributed at least in part to perturbed TPO/Mpl and SCF/Kit signaling.
Figure 5. Signaling defects in Jak2-deficient LSK cells.
(A) Lin− BM cells from control or Jak2-deficient mice were starved for 1 hour in serum-free media at 37°C before they were either left untreated or stimulated with 50ng/ml Tpo for the indicated time. Cells were fixed, permeabilized, and stained with appropriately conjugated antibodies against LSK surface markers and Alexa488 conjugated phospho-stat5, phospho-Erk1/2 or phospho-Akt antibodies. Representative histograms are shown on the top panels, and the fold change in mean fluorescent intensity (MFI) in phospho-stat5, phospho-Erk1/2 or phospho-Akt in Jak2-deficient LSK cells are shown in bar graphs (in the bottom panels) as means ± SEM (n=3, *P< 0.05). (B) Representative histograms on Mpl (TPO receptor) expression in control or Jak2-deficient LSK cells are shown (top panel), and the fold change in mean fluorescent intensity (MFI) in Mpl expression from four independent experiments are shown (in the bottom panel) as means ± SEM. No significant (ns) difference was observed in Mpl expression between control and Jak2-deficient LSK cells. (C) Lin− BM cells from control or Jak2-deficient mice were starved for 1 hour in serum-free media at 37°C before they were either left untreated or stimulated with 50ng/ml SCF for the indicated time. Cells were fixed, permeabilized, and stained for LSK surface markers and phospho-stat5, phospho-Erk1/2 or phospho-Akt as in panel A. Representative histograms are shown on the top panels, and the fold change in mean fluorescent intensity (MFI) in phospho-stat5, phospho-Erk1/2 or phospho-Akt in Jak2-deficient LSK cells are shown in bar graphs (in the bottom panels) as means ± SEM (n=3, *P< 0.05). (D) Relative expression of Kit in SLAM-HSC (Lin−Sca1+CD150+CD41−CD48−). Representative histograms are shown on the top panels, and the fold change in mean fluorescent intensity (MFI) in Kit expression is shown (in the bottom panel) as means ± SEM (n=4 in each group, *P< 0.05). Note that Kit expression was significantly reduced in Jak2-deficient HSCs.
Jak2 deficiency impairs multiple pathways responsible for HSC maintenance
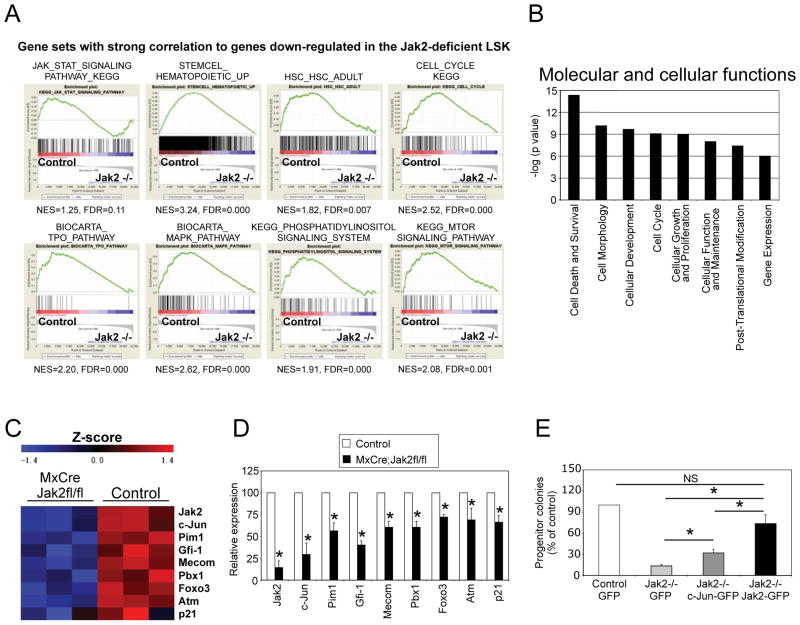
To identify the Jak2-dependent genes and pathways regulating HSC maintenance, we performed global gene expression profiling analyses. We utilized HSC-enriched LSK instead of LT-HSC for microarray analyses since the number of LT-HSC was extremely low in Jak2-deficient mice. Three independent sets of microarray were performed using sorted LSK cells from control and Jak2-deficient mice (15 Jak2-deficient mice were utilized). GSEA [18] on microarray data revealed significant downregulation of HSC-specific genes in Jak2-deficient LSK cells (Fig. 6A and Supplemental Table 1). GSEA also revealed a strong correlation of genes downregulated in the absence of Jak2 with gene sets that identify the Jak/Stat signaling pathway, and various other signaling pathways including TPO, MAPK, PI3 kinase and mTOR pathways (Fig. 6A), which have been implicated in HSC maintenance [22,23,29–32]. GSEA and IPA further revealed enrichment for cell cycle-related and cell death and survival-related genes (Fig. 6A,B and Supplemental Table 1), consistent with the loss of quiescence and increased apoptosis observed in Jak2-deficient LSK. Expression of several genes including c-Jun, Pim-1, Gfi-1, Mecom, Pbx1, Foxo3, Atm and p21Cip1, which were shown to play important roles in the maintenance of quiescence and/or self-renewal of HSCs [20,33–39], was significantly downregulated in Jak2-deficient LSK compared with control LSK (Fig. 6C). Microarray results were further validated by quantitative real-time PCR in sorted LSK cells (Fig. 6D).
Figure 6. Gene expression profile of Jak2-deficient LSK cells.
(A) Gene-set enrichment analyses (GSEAs) were performed on microarray data obtained from control and Jak2-deficient LSK cells. Enrichment plots of selected gene sets from GSEA analysis with normalized enrichment score (NES) and false discovery rate (FDR) are shown. (B) Ingenuity pathway analysis (IPA) of microarray data revealed that most significantly affected molecular and cellular functions associated with Jak2 deficiency were cell death/survival, cell morphology, cellular development, cell cycle, cell growth, cellular function and maintenance, reflecting abnormal cell cycle and survival in Jak2-deficient HSCs. (C) Heat map showing differential expression of a selected list of genes related to HSC maintenance and function in control and Jak2-deficient LSK cells. (D) Relative expression of Jak2, c-Jun, Pim1, Gfi-1, Mecom, Pbx1, Foxo3, Atm, p21 mRNA was determined by quantitative real-time PCR and normalized with 18S expression. Asterisks indicate significant differences by Student t test with P< 0.05. (E) Ectopic expression of c-Jun only partially rescues the defects in hematopoietic progenitor colony formation in Jak2-deficient LSK cells. Lin− BM cells from control or Jak2-deficient mice were transduced with lentiviruses expressing GFP alone or c-Jun-IRES-GFP or Jak2-IRES-GFP in StemSpan H3000 plus BIT9500 medium (StemCell Technologies) containing 50ng/ml SCF and 50ng/ml TPO. GFP-positive LSK cells were sorted using FACS and plated (1 × 104 cells/dish) in methylcellulose medium containing complete cytokines (M3434; StemCell Technologies). Progenitor colonies were counted 7–8 days after plating. Results are expressed as percentage of controls (n=3; *P< 0.05). Note that ectopic expression of wild-type Jak2 almost completely rescued whereas c-Jun expression only partially rescued the defects in hematopoietic progenitor colony formation in Jak2-deficient LSK cells.
Since c-Jun was one of the most highly downregulated genes in Jak2-deficient LSK, we asked if ectopic expression of c-Jun could rescue the defects in Jak2-deficient HSC using hematopoietic progenitor colony formation assay. Whereas wild-type Jak2 expression almost completely rescued the defects in progenitor colony formation, overexpression of c-Jun only partially (although significantly) rescued the defects in progenitor colony formation in Jak2-deficient LSK cells (Fig. 6E). This suggests that multiple genes and pathways related to HSC maintenance and function are perturbed in the absence of Jak2.
DISCUSSION
Although Jak2 has been studied in various cell lines and tissues, genetic data that directly address the role of Jak2 in HSC maintenance and function are not available. In this report, we demonstrate that Jak2 plays a crucial role in the maintenance and function of adult HSCs. Previous studies showed that germ-line deletion of Jak2 perturbed fetal liver erythropoiesis leading to embryonic lethality in mice [3,4]. Park et al. showed that tamoxifen-induced deletion of Jak2 in the whole body of young adult animals impaired erythropoiesis and thrombopoiesis [5]. In this study, we developed MxCre;Jak2fl/fl mice to conditionally delete Jak2 in hematopoietic compartments of adult animals. We observed that deletion of Jak2 resulted in BM aplasia with severe anemia and thrombocytopenia in adult animals (Fig. 1 and Supplemental Fig. S1). In contrast to the previous report by Park et al., in which tamoxifen-induced deletion of Jak2 in young adult animals resulted in only 20% mortality [5], we observed that hematopoietic deletion of Jak2 in adult animals results in 100% mortality (median survival 36 days after induction of Jak2 deletion) (Fig. 1B). The lower rate of mortality observed in tamoxifen-induced adult Jak2 mutant mice was due to incomplete deletion and subsequent repopulation with Jak2-expressing hematopoietic cells [5]. Our data reveal a more profound requirement for Jak2, as complete deletion of Jak2 leads to dramatic loss of HSCs/progenitors in adult animals (Fig. 1D–F).
Assessment of HSC functions using non-competitive and competitive BM transplantation assays reveal that Jak2-deficient HSCs are severely defective in reconstituting hematopoiesis in recipient animals (Fig. 2A–E). Notably, Jak2-deficeint BM cells failed to reconstitute hematopoiesis in recipient animals even when transplanted 20-times more mutant BM cells than control BM cells (Fig. 2A). Also, Jak2-deficient HSCs were unable to compete with WT HSCs and there was rapid loss of hematopoietic progenitors/precursors derived from the Jak2-deficient HSCs even when 10-times more Jak2-deficient BM cells were used in competitive transplantation assays (Fig. 2B, 2C). These data suggest an intrinsic functional defect in Jak2-deficient HSCs.
Most HSCs usually remain in the quiescent state (G0) to prevent HSC exhaustion. Flow cytometric data indicate that Jak2-deficient LSK cells are driven out of quiescence into the cell cycle (Fig. 3A, 3B). Mutant HSCs probably enter into the cell cycle in an attempt to respond to the rapid depletion of hematopoietic cells in Jak2-deficient mice. Increased rate of apoptosis is also evident in Jak2-deficient LSK cells (Fig. 3C). Thus, rapid loss of HSCs in Jak2-deficient mice is possibly due to disruption of HSC quiescence and increased apoptosis.
Aberrant increase in ROS has been associated with disruption of HSC quiescence [37,38,40]. In our experiments, ROS levels were significantly elevated in Jak2-deficient LSK cells (Fig. 4A). It has been shown that elevation of ROS levels induces HSC-specific p38 MAPK activation in Atm-deficient mice [21]. We also observed significant (~ 3 fold) activation of p38 MAPK in Jak2-deficient LSK cells compared with control LSK cells (Fig. 4B). Furthermore, NAC treatment significantly improved the function of Jak2-deficient HSCs, as revealed by significantly increased reconstitution of hematopoiesis in recipient animals transplanted with NAC-treated Jak2-deficient BM BM cells (Fig. 4C). However, NAC treatment (either ex vivo or in vivo) did not completely rescue the Jak2-deficient HSC phenotype, suggesting that all phenotypic manifestations of Jak2 deficiency are not the consequence of increased ROS in the HSC compartment.
Previous studies using cultured cell lines show that Jak2 binds to Mpl and Kit, mediating signaling downstream of TPO and SCF [27,28]. TPO and SCF are required for HSC maintenance [22–26]. We observed defective Erk and Akt activation in Jak2-deficient LSK cells in response to TPO and SCF (Fig. 5A, 5C). Disruption of ErK and Akt signaling may contribute to the HSC phenotype in Jak2-deficient mice, as Erk1/2 and Akt1/2 have been shown to play roles in the maintenance of adult HSCs [29–31]. Interestingly, whereas Mpl expression was not affected by Jak2 deficiency, Kit expression was markedly reduced in Jak2-deficient HSCs (Fig. 5B, 5D). Loss of Kit expression may also contribute to decreased quiescence and increased apoptosis in Jak2-deficient HSCs since Kit is required for HSC quiescence and survival [24,41]. Thus, perturbation of TPO/Mpl and SCF/Kit signaling may contribute to the Jak2-deficient HSC phenotype. However, the HSC phenotype in TPO/MPL or SCF/Kit-deficient mice [22,23,25,41] is less severe than that observed in Jak2-deficient mice, suggesting that combined defects in multiple pathways contribute to the severe HSC phenotype in Jak2-deficient mice.
Gene expression analyses reveal that multiple stem cell maintenance programs are perturbed in the absence of Jak2, including genes associated with Jak/Stat, TPO, MAPK, PI3 kinase and mTOR signaling pathways. GSEA and IPA also reveal that HSC cell cycle and cell death/survival-related genes are significantly altered in Jak2-deficient LSK (Fig. 6A, 6B), reflecting abnormal cell cycle status and loss of quiescence in Jak2-deficient HSCs. Expression of c-Jun, Pim-1, Gfi-1, Mecom, Pbx1, Foxo3, Atm and p21Cip1, which have been implicated in HSC quiescence, survival and/or self-renewal [20,33–39], is significantly downregulated in Jak2-deficient LSK cells (Fig. 6C, and 6D). Ectopic expression of c-Jun only partially rescued the defects in hematopoietic progenitor colony formation in Jak2-deficient LSK (Fig. 6E), suggesting that additional genes and pathways downstream of Jak2 are involved in HSC maintenance and function. It has been shown previously that Atm or Foxo3 deficiency increases ROS levels and decreases the life span of HSCs by disrupting HSC quiescence [37,38,40]. It is thus possible that reduced expression of Atm and Foxo3 may contribute to the elevated ROS levels and loss of quiescence in Jak2-deficient LSK cells. p21 regulates HSC quiescence [39], whereas pim1 is important for HSC survival [34]. Pbx1 and Gfi-1 have been shown to regulate HSC quiescence [20,35], whereas Mecom is required for HSC self-renewal [36]. Reduced expression of several of these genes may contribute to exhaustion of HSCs in Jak2-deficient mice. Thus, abnormal signaling and downregulation of multiple genes related to HSC maintenance and function likely contribute to severe HSC phenotype in Jak2-deficient mice. Further work is needed to define how Jak2 regulates the expression of these genes.
CONCLUSION
In conclusion, we demonstrate that Jak2 plays an essential role in the maintenance and normal function of adult HSCs. The discovery of JAK2V617F mutation in MPNs has led to the development of Jak2 inhibitors. However, patients treated with Jak2 inhibitors show significant hematopoietic toxicities [13–15]. Current Jak2 inhibitors cannot distinguish between wild type and mutant Jak2; so both wild type and mutant Jak2 are inhibited by Jak2 inhibitor treatment. Since Jak2 is critical for the maintenance of normal hematopoiesis, effective treatment of MPNs would require specific targeting of the mutant Jak2 or pathways downstream of Jak2V617F that are required for induction of MPNs. Such therapeutic approaches will minimize hematopoietic toxicities in treating patients with JAK2V617F mutation.
Supplementary Material
Acknowledgments
We thank Dr. Frank Middleton for expert help with microarray data analysis and Dr. Emmanuelle Passegue for lentiviral c-Jun construct. We also thank Lisa Phelps and Karen Gentile for assistance with FACS sorting and microarray processing. This work was supported in parts by grants from the Leukemia & Lymphoma Society and the NIH (R01 HL095685) awarded to G.M. G.M. is a Scholar of the Leukemia & Lymphoma Society.
Footnotes
Author Contributions:
H.A. performed research, analyzed data and wrote the manuscript; S.A. performed research; R.E.H performed histopathologic analysis; K-U.W. and K.S. generated and maintained the Jak2 floxed mice; G.M. designed the research, analyzed data, and wrote the manuscript.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
References
- 1.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 2.Rane SG, Reddy EP. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 2002;21:3334–3358. doi: 10.1038/sj.onc.1205398. [DOI] [PubMed] [Google Scholar]
- 3.Parganas E, Wang D, Stravopodis D, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- 4.Neubauer H, Cumano A, Müller M, et al. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 5.Park SO, Wamsley HL, Bae K, et al. Conditional deletion of Jak2 reveals an essential role in hematopoiesis throughout mouse ontogeny: implications for Jak2 inhibition in humans. PLoS One. 2013;8:e59675. doi: 10.1371/journal.pone.0059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signaling causes polycythemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 8.Levine RL, Wadleigh M, cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Eng J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 10.Zhao R, Xing S, Li Z, et al. Identification of an Acquired JAK2 Mutation in Polycythemia Vera. J Biol Chem. 2005;280:22788–22792. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6:372–375. [PubMed] [Google Scholar]
- 12.Levine RL, Gilliland DG. Myeloproliferative disorders. Blood. 2008;112:2190–2198. doi: 10.1182/blood-2008-03-077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos FP, Kantarjian HM, Jain N, et al. Phase 2 study of CEP–701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115:1131–1136. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pardanani A, Gotlib JR, Jamieson C, et al. Safety and Efficacy of TG101348, a Selective JAK2 Inhibitor, in Myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tefferi A, Litzow MR, Pardanani A. Long-term outcome of treatment with ruxolitinib in myelofibrosis. N Engl J Med. 2011;365:1455–1457. doi: 10.1056/NEJMc1109555. [DOI] [PubMed] [Google Scholar]
- 16.Krempler A, Qi Y, Triplett AA, Zhu J, Rui H, Wagner KU. Generation of a conditional knockout allele for the Janus kinase 2 (Jak2) gene in mice. Genesis. 2004;40:52–57. doi: 10.1002/gene.20063. [DOI] [PubMed] [Google Scholar]
- 17.Kühn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pronk CJ, Rossi DJ, Månsson R, et al. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2:484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara H, Arai F, Hosokawa K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 23.Qian H, Buza-Vidas N, Hyland CD, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Domen J, Weissman IL. Hematopoietic stem cells need two signals to prevent apoptosis; BCL-2 can provide one of these, Kitl/c-Kit signaling the other. J Exp Med. 2000;192:1707–1718. doi: 10.1084/jem.192.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding L, Saunders T, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang CC, Lodish H. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol. 2008;15:307–311. doi: 10.1097/MOH.0b013e3283007db5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. 1999;274:13480–13484. doi: 10.1074/jbc.274.19.13480. [DOI] [PubMed] [Google Scholar]
- 28.Weiler SR, Mou S, DeBerry CS, et al. JAK2 is associated with the c-kit proto-oncogene product and is phosphorylated in response to stem cell factor. Blood. 1996;87:3688–3693. [PubMed] [Google Scholar]
- 29.Staser K, Park SJ, Rhodes SD, et al. Normal hematopoiesis and neurofibromin-deficient myeloproliferative disease require Erk. J Clin Invest. 2013;123:329–334. doi: 10.1172/JCI66167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan G, Gu S, Neel BG. Erk1 and Erk2 are required for maintenance of hematopoietic stem cells and adult hematopoiesis. Blood. 2013;121:3594–3598. doi: 10.1182/blood-2012-12-476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalaitzidis D, Sykes SM, Wang Z, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An N, Lin YW, Mahajan S, et al. Pim1 Serine/Threonine Kinase Regulates the Number and Functions of Murine Hematopoietic Stem Cells. Stem Cells. 2013;31:1202–1212. doi: 10.1002/stem.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hock H, Hamblen MJ, Rooke HM, et al. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431:1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Stehling-Sun S, Lezon-Geyda K, et al. PR-domain-containing Mds1-Evi1 is critical for long-term hematopoietic stem cell function. Blood. 2011;118:3853–3861. doi: 10.1182/blood-2011-02-334680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto K, Arai KY, Naka K, et al. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 39.Cheng T, Rodrigues N, Shen H, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 40.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Thorén LA, Liuba K, Bryder D, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.