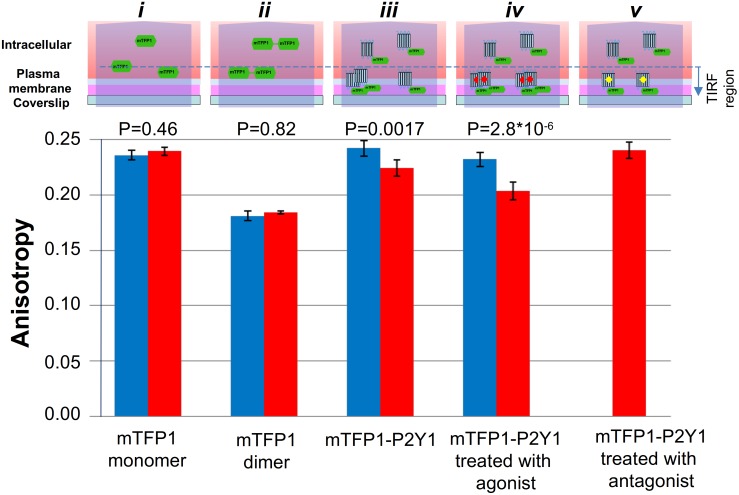
Figure 7. Measured anisotropy in different illumination modes, for analysis receptor assemblies in the cell membrane.
Anisotropy, measured in the TIRF and EPI modes, of mTFP1 alone (i), or in a pair joined by a 20-aminoacid linker (ii), or attached to P2Y1R without (iii), or with (iv) the 10 µM2-MeSATP agonist or the 1 µM P2Y1R-selective antagonist MRS-2500 present (v). The diagrams above the bar plots represent the schematic interpretation of the anisotropy results in the TIRF and EPI illumination modes. (i) The monomeric mTFP1 fluorescent protein, expressed alone, localizes only in the interior of the cell, since it does not translocate into the plasma membrane or form dimers. The measured anisotropy value was found to be independent of the illumination modes. (ii) In control measurements with the mTFP1-20aa-mTFP1 construct, the mTFP1 homo-dimer is intracellularly located and showed much-reduced anisotropy due to homo-FRET between the two linked chromophores, under both illuminations. (iii) The P2Y1R was N-terminally tagged with mTFP1 and expressed. In the EPI mode the intracellular, monomeric mTFP-P2Y1R fusion protein produces the main component of the anisotropy, but in the TIRF mode its observed location is strongly restricted to sites within and near the membrane. (iv) Upon agonist treatment, the labeled P2Y1 receptors readily form dimers in the plasma membrane with almost 100% efficiency. (v) Application of a highly potent P2Y1R selective antagonist, completely reverses the agonist-induced decrease in anisotropy in the TIRF mode, confirming the P2Y1R involvement.